Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia
- PMID: 25977177
- PMCID: PMC4610712
- DOI: 10.1111/jns.12114
Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia
Abstract
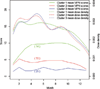
Vincristine, a critical component of combination chemotherapy treatment for pediatric acute lymphoblastic leukemia (ALL), can lead to vincristine-induced peripheral neuropathy (VIPN). Longitudinal VIPN assessments were obtained over 12 months from newly diagnosed children with ALL (N = 128) aged 1-18 years who received vincristine at one of four academic children's hospitals. VIPN assessments were obtained using the Total Neuropathy Score-Pediatric Vincristine (TNS©-PV), National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE©), Balis© grading scale, and Pediatric Neuropathic Pain Scale©-Five (PNPS©-5). Of children who provided a full TNS©-PV score, 85/109 (78%) developed VIPN (TNS©-PV ≥4). Mean TNS©-PV, grading scale, and pain scores were low. CTCAE©-derived grades 3 and 4 sensory and motor VIPN occurred in 1.6%/0%, and 1.9%/0% of subjects, respectively. VIPN did not resolve in months 8-12 despite decreasing dose density. VIPN was worse in older children. Partition cluster analysis revealed 2-3 patient clusters; one cluster (n = 14) experienced severe VIPN. In this population, VIPN occurs more commonly than previous research suggests, persists throughout the first year of treatment, and can be severe.
Keywords: acute lymphoblastic leukemia; children; patterns; severity; vincristine-induced peripheral neuropathy.
© 2015 Peripheral Nerve Society.
Figures


References
-
- American Cancer Society. [Accessed October 25, 2013];Childhood leukemia. 2013 Updated 2013. http://www.cancer.org.
-
- American Cancer Society. [Accessed February 26, 2015];Cancer Facts & Figures 2014. 2014 http://www.cancer.org.
-
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. - PubMed
-
- Bisogno G, Ferrari A, Bergeron C, Scagnellato A, Prete A, Alaggio R, Casanova M, D’Angelo P, Di Cataldo A, Carli M. The IVADo regimen - a pilot study with ifosfamide, vincristine, actinomycin D, and doxorubicin in children with metastatic soft tissue sarcoma: a pilot study of behalf of the European Pediatric Soft Tissue Sarcoma Study Grou. Cancer. 2005;103:1719–1724. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

