Renal Production, Uptake, and Handling of Circulating αKlotho
- PMID: 25977312
- PMCID: PMC4696570
- DOI: 10.1681/ASN.2014101030
Renal Production, Uptake, and Handling of Circulating αKlotho
Abstract
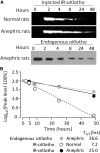
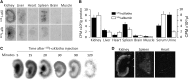
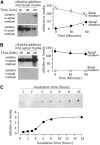
αKlotho is a multifunctional protein highly expressed in the kidney. Soluble αKlotho is released through cleavage of the extracellular domain from membrane αKlotho by secretases to function as an endocrine/paracrine substance. The role of the kidney in circulating αKlotho production and handling is incompletely understood, however. Here, we found higher αKlotho concentration in suprarenal compared with infrarenal inferior vena cava in both rats and humans. In rats, serum αKlotho concentration dropped precipitously after bilateral nephrectomy or upon treatment with inhibitors of αKlotho extracellular domain shedding. Furthermore, the serum half-life of exogenous αKlotho in anephric rats was four- to five-fold longer than that in normal rats, and exogenously injected labeled recombinant αKlotho was detected in the kidney and in urine of rats. Both in vivo (micropuncture) and in vitro (proximal tubule cell line) studies showed that αKlotho traffics from the basal to the apical side of the proximal tubule via transcytosis. Thus, we conclude that the kidney has dual roles in αKlotho homeostasis, producing and releasing αKlotho into the circulation and clearing αKlotho from the blood into the urinary lumen.
Keywords: Cell & Transport Physiology; Nephrectomy; Transcytosis; [REMOVED HYPERLINK FIELD]Klotho; distal tubule; renal proximal tubule cell.
Copyright © 2016 by the American Society of Nephrology.
Figures



References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 - PubMed
-
- Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI: Molecular cloning and expression analyses of mouse βKlotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

