Gastrin stimulates MMP-1 expression in gastric epithelial cells: putative role in gastric epithelial cell migration
- PMID: 25977510
- PMCID: PMC4504956
- DOI: 10.1152/ajpgi.00084.2015
Gastrin stimulates MMP-1 expression in gastric epithelial cells: putative role in gastric epithelial cell migration
Abstract
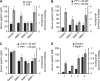
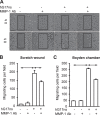
The pyloric antral hormone gastrin plays a role in remodeling of the gastric epithelium, but the specific targets of gastrin that mediate these effects are poorly understood. Glandular epithelial cells of the gastric corpus express matrix metalloproteinase (MMP)-1, which is a potential determinant of tissue remodeling; some of these cells express the CCK-2 receptor at which gastrin acts. We have now examined the hypothesis that gastrin stimulates expression of MMP-1 in the stomach. We determined MMP-1 transcript abundance in gastric mucosal biopsies from Helicobacter pylori negative human subjects with normal gastric mucosal histology, who had a range of serum gastrin concentrations due in part to treatment with proton pump inhibitors (PPI). The effects of gastrin were studied on gastric epithelial AGS-GR cells using Western blot and migration assays. In human subjects with increased serum gastrin due to PPI usage, MMP-1 transcript abundance was increased 2-fold; there was also increased MMP-7 transcript abundance but not MMP-3. In Western blots, gastrin increased proMMP-1 abundance, as well that of a minor band corresponding to active MMP-1, in the media of AGS-GR cells, and the response was mediated by protein kinase C and p42/44 MAP kinase. There was also increased MMP-1 enzyme activity. Gastrin-stimulated AGS-GR cell migration in both scratch wound and Boyden chamber assays was inhibited by MMP-1 immunoneutralization. We conclude that MMP-1 expression is a target of gastrin implicated in mucosal remodeling.
Keywords: MMP-1; gastric epithelium; gastrin.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Baba M, Itoh K, Tatsuta M. Glycine-extended gastrin induces matrix metalloproteinase-1- and -3-mediated invasion of human colon cancer cells through type I collagen gel and Matrigel. Int J Cancer 111: 23–31, 2004. - PubMed
-
- Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion. Trends Pharmacol Sci 8: 486–490, 1987.
-
- Bodger K, Ahmed S, Pazmany L, Pritchard DM, Micheal A, Khan AL, Dimaline R, Dockray GJ, Varro A. Altered gastric corpus expression of tissue inhibitors of metalloproteinases in human and murine Helicobacter infection. J Clin Pathol 61: 72–78, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

