Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation
- PMID: 25977584
- PMCID: PMC4481597
- DOI: 10.1182/blood-2015-02-628354
Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation
Abstract
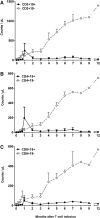
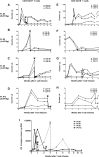
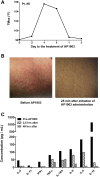
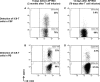
To test the feasibility of a single T-cell manipulation to eliminate alloreactivity while sparing antiviral and antitumor T cells, we infused 12 haploidentical hematopoietic stem cell transplant patients with increasing numbers of alloreplete haploidentical T cells expressing the inducible caspase 9 suicide gene (iC9-T cells). We determined whether the iC9-T cells produced immune reconstitution and if any resultant graft-versus-host disease (GVHD) could be controlled by administration of a chemical inducer of dimerization (CID; AP1903/Rimiducid). All patients receiving >10(4) alloreplete iC9-T lymphocytes per kilogram achieved rapid reconstitution of immune responses toward 5 major pathogenic viruses and concomitant control of active infections. Four patients received a single AP1903 dose. CID infusion eliminated 85% to 95% of circulating CD3(+)CD19(+) T cells within 30 minutes, with no recurrence of GVHD within 90 days. In one patient, symptoms and signs of GVHD-associated cytokine release syndrome (CRS-hyperpyrexia, high levels of proinflammatory cytokines, and rash) resolved within 2 hours of AP1903 infusion. One patient with varicella zoster virus meningitis and acute GVHD had iC9-T cells present in the cerebrospinal fluid, which were reduced by >90% after CID. Notably, virus-specific T cells recovered even after AP1903 administration and continued to protect against infection. Hence, alloreplete iC9-T cells can reconstitute immunity posttransplant and administration of CID can eliminate them from both peripheral blood and the central nervous system (CNS), leading to rapid resolution of GVHD and CRS. The approach may therefore be useful for the rapid and effective treatment of toxicities associated with infusion of engineered T lymphocytes. This trial was registered at www.clinicaltrials.gov as #NCT01494103.
© 2015 by The American Society of Hematology.
Figures


References
-
- André-Schmutz I, Le Deist F, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360(9327):130–137. - PubMed
-
- Palma J, Salas L, Carrión F, et al. Haploidentical stem cell transplantation for children with high-risk leukemia. Pediatr Blood Cancer. 2012;59(5):895–901. - PubMed
-
- Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–1193. - PubMed
-
- Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

