Topical Developments in High-Field Dynamic Nuclear Polarization
- PMID: 25977588
- PMCID: PMC4428548
- DOI: 10.1002/ijch.201300126
Topical Developments in High-Field Dynamic Nuclear Polarization
Abstract
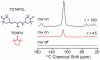
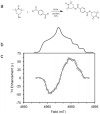
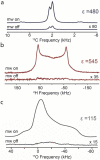
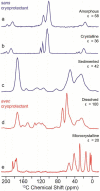
We report our recent efforts directed at improving high-field DNP experiments. We investigated a series of thiourea nitroxide radicals and the associated DNP enhancements ranging from ε = 25 to 82 that demonstrate the impact of molecular structure on performance. We directly polarized low-gamma nuclei including 13C, 2H, and 17O using trityl via the cross effect. We discuss a variety of sample preparation techniques for DNP with emphasis on the benefit of methods that do not use a glass-forming cryoprotecting matrix. Lastly, we describe a corrugated waveguide for use in a 700 MHz / 460 GHz DNP system that improves microwave delivery and increases enhancements up to 50%.
Keywords: DNP; NMR; cross-effect; cryoprotection; polarizing agent; radicals.
Figures
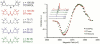






Similar articles
-
Verdazyl-Based Radicals for High-Field Dynamic Nuclear Polarization NMR.J Am Chem Soc. 2025 Mar 5;147(9):7293-7304. doi: 10.1021/jacs.4c13374. Epub 2025 Feb 21. J Am Chem Soc. 2025. PMID: 39982131
-
Optimization of dynamic nuclear polarization experiments in aqueous solution at 15 MHz/9.7 GHz: a comparative study with DNP at 140 MHz/94 GHz.Phys Chem Chem Phys. 2010 Jun 14;12(22):5893-901. doi: 10.1039/c002814m. Epub 2010 May 8. Phys Chem Chem Phys. 2010. PMID: 20454734
-
Efficient Dynamic Nuclear Polarization at 800 MHz/527 GHz with Trityl-Nitroxide Biradicals.Angew Chem Int Ed Engl. 2015 Sep 28;54(40):11770-4. doi: 10.1002/anie.201504292. Epub 2015 Aug 12. Angew Chem Int Ed Engl. 2015. PMID: 26268156 Free PMC article.
-
Dynamic nuclear polarization at 700 MHz/460 GHz.J Magn Reson. 2012 Nov;224:1-7. doi: 10.1016/j.jmr.2012.08.002. Epub 2012 Aug 14. J Magn Reson. 2012. PMID: 23000974 Free PMC article.
-
Dynamic Nuclear Polarization enhanced NMR at 187 GHz/284 MHz using an Extended Interaction Klystron amplifier.J Magn Reson. 2016 Apr;265:77-82. doi: 10.1016/j.jmr.2016.01.021. Epub 2016 Feb 2. J Magn Reson. 2016. PMID: 26867091
Cited by
-
Combining DNP NMR with segmental and specific labeling to study a yeast prion protein strain that is not parallel in-register.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3642-3647. doi: 10.1073/pnas.1619051114. Epub 2017 Mar 22. Proc Natl Acad Sci U S A. 2017. PMID: 28330994 Free PMC article.
-
Dynamic nuclear polarization of (1)H, (13)C, and (59)Co in a tris(ethylenediamine)cobalt(III) crystalline lattice doped with Cr(III).J Am Chem Soc. 2014 Aug 20;136(33):11716-27. doi: 10.1021/ja5044374. Epub 2014 Aug 8. J Am Chem Soc. 2014. PMID: 25069794 Free PMC article.
-
Efficient DNP NMR of membrane proteins: sample preparation protocols, sensitivity, and radical location.J Biomol NMR. 2016 Mar;64(3):223-37. doi: 10.1007/s10858-016-0023-3. Epub 2016 Feb 12. J Biomol NMR. 2016. PMID: 26873390 Free PMC article.
-
17O MAS NMR Correlation Spectroscopy at High Magnetic Fields.J Am Chem Soc. 2017 Dec 13;139(49):17953-17963. doi: 10.1021/jacs.7b08989. Epub 2017 Nov 30. J Am Chem Soc. 2017. PMID: 29111706 Free PMC article.
-
(17)O NMR Investigation of Water Structure and Dynamics.J Phys Chem B. 2016 Aug 18;120(32):7851-8. doi: 10.1021/acs.jpcb.6b05755. Epub 2016 Aug 9. J Phys Chem B. 2016. PMID: 27454747 Free PMC article.
References
-
- Harris RK. Applications of Solid-State NMR to Pharmaceutical Polymorphism and Related Matters*. Journal of Pharmacy and Pharmacology. 2007;59(2):225–239. - PubMed
-
- Vogt FG. Evolution of Solid-State NMR in Pharmaceutical Analysis. Future Medicinal Chemistry. 2010;2(6):915–921. - PubMed
-
- Brown SP. Applications of High-Resolution H-1 Solid-State NMR. Solid State Nuclear Magnetic Resonance. 2012;41:1–27. - PubMed
-
- McDermott A. Structure and Dynamics of Membrane Proteins by Magic Angle Spinning Solid-State NMR. Annual Review of Biophysics. 2009;38:385–403. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
