Hepatitis infection in the treatment of opioid dependence and abuse
- PMID: 25977607
- PMCID: PMC4395041
- DOI: 10.4137/sart.s580
Hepatitis infection in the treatment of opioid dependence and abuse
Abstract
Many new and existing cases of viral hepatitis infections are related to injection drug use. Transmission of these infections can result directly from the use of injection equipment that is contaminated with blood containing the hepatitis B or C virus or through sexual contact with an infected individual. In the latter case, drug use can indirectly contribute to hepatitis transmission through the dis-inhibited at-risk behavior, that is, unprotected sex with an infected partner. Individuals who inject drugs are at-risk for infection from different hepatitis viruses, hepatitis A, B, or C. Those with chronic hepatitis B virus infection also face additional risk should they become co-infected with hepatitis D virus. Protection from the transmission of hepatitis viruses A and B is best achieved by vaccination. For those with a history of or who currently inject drugs, the medical management of viral hepatitis infection comprising screening, testing, counseling and providing care and treatment is evolving. Components of the medical management of hepatitis infection, for persons considering, initiating, or receiving pharmacologic therapy for opioid addiction include: testing for hepatitis B and C infections; education and counseling regarding at-risk behavior and hepatitis transmission, acute and chronic hepatitis infection, liver disease and its care and treatment; vaccination against hepatitis A and B infection; and integrative primary care as part of the comprehensive treatment approach for recovery from opioid abuse and dependence. In addition, participation in a peer support group as part of integrated medical care enhances treatment outcomes. Liver disease is highly prevalent in patient populations seeking recovery from opioid addiction or who are currently receiving pharmacotherapy for opioid addiction. Pharmacotherapy for opioid addiction is not a contraindication to evaluation, care, or treatment of liver disease due to hepatitis virus infection. Successful pharmacotherapy for opioid addiction stabilizes patients and improves patient compliance to care and treatment regimens as well as promotes good patient outcomes. Implementation and integration of effective hepatitis prevention programs, care programs, and treatment regimens in concert with the pharmacological therapy of opioid addiction can reduce the public health burdens of hepatitis and injection drug use.
Keywords: hepatitis; medication assisted treatment; methadone; substance abuse treatment.
Figures
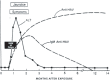

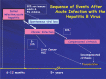


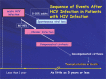

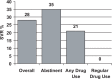
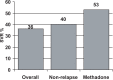

References
-
- AASLD, (American Association for the Study of Liver Disease) Chronic Hepatitis B AASLD Practice Guidelines. 2003. Available at: https://www.aasld.org/eweb/docs/chronichep_B.pdf.
-
- AASLD, (American Association for the Study of Liver Disease) Diagnosis Management Treatment of Hepatitis C, AASLD Practice Guideline. Hepatology. 2004;39:1147–71. - PubMed
-
- Afdhal NH. Biopsy or biomarkers: is there a gold standard for diagnosis of liver fibrosis? Clin Chem. 2004;50:1299–300. - PubMed
-
- Afdhal NH, Godofsky E, Dienstag J, et al. Final Phase I/II trial results for NM283, a new polymerase inhibitor for hepatitis C: Antiviral efficacy and tolerance in patients with HCV-1 infection, including previous interferon failures; Presentation at the American Association for the Study of Liver Disease 55th Annual Meeting and Postgraduate Course; October 29, 2004; Boston, Massachusetts. 2004. Abstract LB-03.
-
- Aitken CK, Kerger M, Crofts N. Peer-delivered hepatitis C testing and counseling: a means of improving the health of injection drug users. Drug Alcohol Rev. 2002;21:33–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

