The reactions of 2-ethoxymethylidene-3-oxo esters and their analogues with 5-aminotetrazole as a way to novel azaheterocycles
- PMID: 25977712
- PMCID: PMC4419505
- DOI: 10.3762/bjoc.11.44
The reactions of 2-ethoxymethylidene-3-oxo esters and their analogues with 5-aminotetrazole as a way to novel azaheterocycles
Abstract
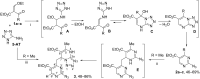
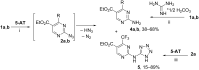
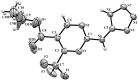
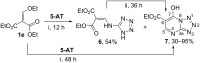
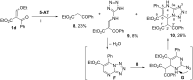
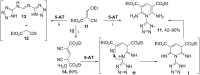
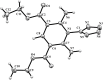
The interaction of 2-ethoxymethylidene-3-oxo esters and their analogues with 5-aminotetrazole is an efficient synthetic approach to novel azaheterocycles. 2-Ethoxymethylidene-3-oxo esters bearing alkyl substituents react with 5-aminotetrazole to form ethyl 2-azido-4-alkylpyrimidine-5-carboxylates which are capable of subsequent nucleophilic substitution. The use of diethyl 2-ethoxymethylidenemalonate in this reaction resulted in ethyl 7-hydroxytetrazolo[1,5-a]pyrimidine-6-carboxylate, while ethyl 2-ethoxymethylidenecyanoacetate yielded 5-[2,6-diamino-3,5-bis(ethoxycarbonyl)pyridinium-1-yl]tetrazol-1-ide through an alternative pathway. Ethyl 2-benzoyl-3-ethoxyprop-2-enoate reacted with 5-aminotetrazole by two reaction routes to form ethyl 2-benzoyl-3-(1H-tetrazol-5-ylamino)prop-2-enoate and ethyl 7-(1-ethoxy-1,3-dioxo-3-phenylpropan-2-yl)-5-phenyl-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate.
Keywords: 2-ethoxymethylidene-3-oxo esters; 5-aminotetrazole; cyclisation; diethyl 2-ethoxymethylidenemalonate; ethyl 2-ethoxymethylidenecyanoacetate.
Figures
References
-
- Kudyakova Yu S, Bazhin D N, Goryaeva M V, Burgart Y V, Saloutin V I. Russ Chem Rev. 2014;83:120–142. doi: 10.1070/RC2014v083n02ABEH004388. - DOI
-
- Ram V J, Pratibha S, Singh S K, Kandpal M, Tekwani B L. Bioorg Med Chem Lett. 1997;7:1087–1090. doi: 10.1016/S0960-894X(97)00166-2. - DOI
-
- Ruisi G, Canfora L, Bruno G, Rotondo A, Mastropietro T F, Debbia E A, Girasolo M A, Megna B. J Organomet Chem. 2010;695:546–551. doi: 10.1016/j.jorganchem.2009.11.019. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
