Diastereoselective and enantioselective conjugate addition reactions utilizing α,β-unsaturated amides and lactams
- PMID: 25977728
- PMCID: PMC4419509
- DOI: 10.3762/bjoc.11.60
Diastereoselective and enantioselective conjugate addition reactions utilizing α,β-unsaturated amides and lactams
Abstract
The conjugate addition reaction has been a useful tool in the formation of carbon-carbon bonds. The utility of this reaction has been demonstrated in the synthesis of many natural products, materials, and pharmacological agents. In the last three decades, there has been a significant increase in the development of asymmetric variants of this reaction. Unfortunately, conjugate addition reactions using α,β-unsaturated amides and lactams remain underdeveloped due to their inherently low reactivity. This review highlights the work that has been done on both diastereoselective and enantioselective conjugate addition reactions utilizing α,β-unsaturated amides and lactams.
Keywords: Michael addition; conjugate addition; α,β-unsaturated amides; α,β-unsaturated lactams.
Figures




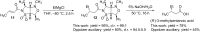
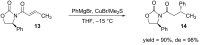


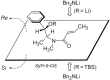





















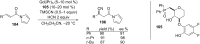

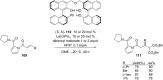









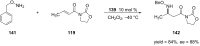









References
-
- Posner G H. In: Organic Reactions. Dauben W G, editor. New York, NY, USA: John Wiley & Sons, Inc.; 1972. pp. 1–114.
-
- Lipshutz B H, Sengupta S. In: Organic Reactions. Paquette L A, editor. New York, NY, USA: John Wiley & Sons, Inc.; 1992. pp. 135–631.
-
- Rossiter B E, Swingle N M. Chem Rev. 1992;92:771–806. doi: 10.1021/cr00013a002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
