Modern brain tumor imaging
- PMID: 25977902
- PMCID: PMC4426283
- DOI: 10.14791/btrt.2015.3.1.8
Modern brain tumor imaging
Abstract
The imaging and clinical management of patients with brain tumor continue to evolve over time and now heavily rely on physiologic imaging in addition to high-resolution structural imaging. Imaging remains a powerful noninvasive tool to positively impact the management of patients with brain tumor. This article provides an overview of the current state-of-the art clinical brain tumor imaging. In this review, we discuss general magnetic resonance (MR) imaging methods and their application to the diagnosis of, treatment planning and navigation, and disease monitoring in patients with brain tumor. We review the strengths, limitations, and pitfalls of structural imaging, diffusion-weighted imaging techniques, MR spectroscopy, perfusion imaging, positron emission tomography/MR, and functional imaging. Overall this review provides a basis for understudying the role of modern imaging in the care of brain tumor patients.
Keywords: Brain neoplasms; Glioblastoma; Glioma; Magnetic resonance imaging; Neuroimaging.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
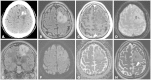
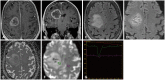
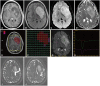
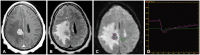
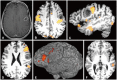
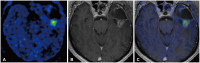
References
-
- Bangiyev L, Rossi Espagnet MC, Young R, et al. Adult brain tumor imaging: state of the art. Semin Roentgenol. 2014;49:39–52. - PubMed
-
- Zulch KJ. Histological typing of tumours of the central nervous system. Geneva: World Health Organization; 1979.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

