Tie-2 regulates the stemness and metastatic properties of prostate cancer cells
- PMID: 25978029
- PMCID: PMC4823056
- DOI: 10.18632/oncotarget.3950
Tie-2 regulates the stemness and metastatic properties of prostate cancer cells
Abstract
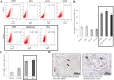
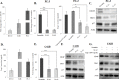
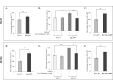
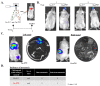
Ample evidence supports that prostate tumor metastasis originates from a rare population of cancer cells, known as cancer stem cells (CSCs). Unfortunately, little is known about the identity of these cells, making it difficult to target the metastatic prostate tumor. Here, for the first time, we report the identification of a rare population of prostate cancer cells that express the Tie-2 protein. We found that this Tie-2High population exists mainly in prostate cancer cell lines that are capable of metastasizing to the bone. These cells not only express a higher level of CSC markers but also demonstrate enhanced resistance to the chemotherapeutic drug Cabazitaxel. In addition, knockdown of the expression of the Tie-2 ligand angiopoietin (Ang-1) led to suppression of CSC markers, suggesting that the Ang-1/Tie-2 signaling pathway functions as an autocrine loop for the maintenance of prostate CSCs. More importantly, we found that Tie-2High prostate cancer cells are more adhesive than the Tie-2Low population to both osteoblasts and endothelial cells. Moreover, only the Tie-2High, but not the Tie-2Low cells developed tumor metastasis in vivo when injected at a low number. Taken together, our data suggest that Tie-2 may play an important role during the development of prostate tumor metastasis.
Keywords: Tie-2; cancer stem cells; metastasis; prostate cancer.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


Similar articles
-
The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches.PLoS One. 2014 Dec 8;9(12):e114530. doi: 10.1371/journal.pone.0114530. eCollection 2014. PLoS One. 2014. PMID: 25485970 Free PMC article.
-
Contextual effect of repression of bone morphogenetic protein activity in prostate cancer.Endocr Relat Cancer. 2013 Nov 4;20(6):861-74. doi: 10.1530/ERC-13-0100. Print 2013 Dec. Endocr Relat Cancer. 2013. PMID: 24042462 Free PMC article.
-
Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway.Int J Mol Sci. 2019 Mar 7;20(5):1164. doi: 10.3390/ijms20051164. Int J Mol Sci. 2019. PMID: 30866453 Free PMC article.
-
A CD90(+) tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer.Cancer Res. 2013 Apr 1;73(7):2322-32. doi: 10.1158/0008-5472.CAN-12-2991. Epub 2013 Feb 4. Cancer Res. 2013. PMID: 23382045
-
Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer.Dev Cell. 2017 Jun 5;41(5):467-480.e3. doi: 10.1016/j.devcel.2017.05.005. Dev Cell. 2017. PMID: 28586644 Free PMC article.
Cited by
-
A Comprehensive Characterization of Stemness in Cell Lines and Primary Cells of Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2022 Sep 14;23(18):10663. doi: 10.3390/ijms231810663. Int J Mol Sci. 2022. PMID: 36142575 Free PMC article.
-
TIE2 Induces Breast Cancer Cell Dormancy and Inhibits the Development of Osteolytic Bone Metastases.Cancers (Basel). 2020 Apr 3;12(4):868. doi: 10.3390/cancers12040868. Cancers (Basel). 2020. PMID: 32260072 Free PMC article.
-
Molecular determinants of prostate cancer metastasis.Oncotarget. 2017 Sep 19;8(50):88211-88231. doi: 10.18632/oncotarget.21085. eCollection 2017 Oct 20. Oncotarget. 2017. PMID: 29152153 Free PMC article. Review.
-
Pericyte-expressed Tie2 controls angiogenesis and vessel maturation.Nat Commun. 2017 Jul 18;8:16106. doi: 10.1038/ncomms16106. Nat Commun. 2017. PMID: 28719590 Free PMC article.
-
Expression of Angiopoietin and VEGF in Cervical Cancer and its Clinical Significance.Open Life Sci. 2018 Dec 31;13:527-532. doi: 10.1515/biol-2018-0063. eCollection 2018 Jan. Open Life Sci. 2018. PMID: 33817123 Free PMC article.
References
-
- Taylor RA, Toivanen R, Risbridger GP. Stem cells in prostate cancer: treating the root of the problem. Endocrine-related cancer. 2010;17:R273–285. - PubMed
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature reviews Cancer. 2001;1:34–45. - PubMed
-
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England journal of medicine. 2006;355:1253–1261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

