Hypoxia attenuates the proinflammatory response in colon cancer cells by regulating IκB
- PMID: 25978030
- PMCID: PMC4653005
- DOI: 10.18632/oncotarget.3961
Hypoxia attenuates the proinflammatory response in colon cancer cells by regulating IκB
Abstract
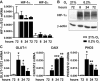
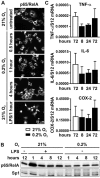
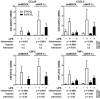
Two main features common to all solid tumors are tissue hypoxia and inflammation, both of which cause tumor progression, metastasis, therapy resistance and increased mortality. Chronic inflammation is associated with increased cancer risk, as demonstrated for inflammatory bowel disease patients developing colon cancer. However, the interplay between hypoxia and inflammation on the molecular level remains to be elucidated. We found that MC-38 mouse colon cancer cells contain functional hypoxic (HIF-1α) and inflammatory (p65/RelA) signaling pathways. In contrast to cells of the myeloid lineage, HIF-1α levels remained unaffected in MC-38 cells treated with LPS, and hypoxia failed to induce NF-κB. A similar regulation of canonical HIF and NF-κB target genes confirmed these results. RNA deep sequencing of HIF-1α and p65/RelA knock-down cells revealed that a surprisingly large fraction of HIF target genes required p65/RelA for hypoxic regulation and a number of p65/RelA target genes required HIF-1α for proinflammatory regulation, respectively. Hypoxia attenuated the inflammatory response to LPS by inhibiting nuclear translocation of p65/RelA independently of HIF-1α, which was associated with enhanced IκBα levels and decreased IKKβ phosphorylation. These data demonstrate that the interaction between hypoxic and inflammatory signaling pathways needs to be considered when designing cancer therapies targeting HIF or NF-κB.
Keywords: NF-κB; inflammatory bowel disease; lipopolysaccharide; tissue oxygenation; tumor hypoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
[Hypoxia/reoxygenation and lipopolysaccharide induced nuclear factor-ΚB and hypoxia-inducible factor-1α signaling pathways in intestinal epithelial cell injury and the interventional effect of emodin].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014 Jun;26(6):409-14. doi: 10.3760/cma.j.issn.2095-4352.2014.06.009. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014. PMID: 24912640 Chinese.
-
Amelioration of hypoxia and LPS-induced intestinal epithelial barrier dysfunction by emodin through the suppression of the NF-κB and HIF-1α signaling pathways.Int J Mol Med. 2014 Dec;34(6):1629-39. doi: 10.3892/ijmm.2014.1965. Epub 2014 Oct 13. Int J Mol Med. 2014. PMID: 25318952
-
NF-κB suppresses HIF-1α response by competing for P300 binding.Biochem Biophys Res Commun. 2011 Jan 28;404(4):997-1003. doi: 10.1016/j.bbrc.2010.12.098. Epub 2010 Dec 25. Biochem Biophys Res Commun. 2011. PMID: 21187066
-
Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation.Trends Biochem Sci. 2005 Jan;30(1):43-52. doi: 10.1016/j.tibs.2004.11.009. Trends Biochem Sci. 2005. PMID: 15653325 Review.
-
Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression.Biomed Pharmacother. 2014 Feb;68(1):1-5. doi: 10.1016/j.biopha.2013.10.013. Epub 2013 Nov 15. Biomed Pharmacother. 2014. PMID: 24286852 Review.
Cited by
-
Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea.Respir Res. 2018 Feb 12;19(1):28. doi: 10.1186/s12931-018-0727-x. Respir Res. 2018. PMID: 29433520 Free PMC article.
-
Role of Oxidative Stress in the Occurrence and Development of Cognitive Dysfunction in Patients with Obstructive Sleep Apnea Syndrome.Mol Neurobiol. 2024 Aug;61(8):5083-5101. doi: 10.1007/s12035-023-03899-3. Epub 2023 Dec 30. Mol Neurobiol. 2024. PMID: 38159196 Review.
-
Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms.Int J Mol Sci. 2021 Oct 2;22(19):10701. doi: 10.3390/ijms221910701. Int J Mol Sci. 2021. PMID: 34639040 Free PMC article. Review.
-
Dietary selenium protects adiponectin knockout mice against chronic inflammation induced colon cancer.Cancer Biol Ther. 2017 Apr 3;18(4):257-267. doi: 10.1080/15384047.2016.1276130. Epub 2017 Jan 3. Cancer Biol Ther. 2017. PMID: 28045589 Free PMC article.
-
The study of JAZF1-mediated apoptosis of decidual stromal cells by activating the NF-κB signaling pathway in spontaneous preterm birth.BMC Pregnancy Childbirth. 2025 Aug 27;25(1):891. doi: 10.1186/s12884-025-07983-5. BMC Pregnancy Childbirth. 2025. PMID: 40866818 Free PMC article.
References
-
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
-
- Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. - PubMed
-
- Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

