covR Mediated Antibiofilm Activity of 3-Furancarboxaldehyde Increases the Virulence of Group A Streptococcus
- PMID: 25978065
- PMCID: PMC4433207
- DOI: 10.1371/journal.pone.0127210
covR Mediated Antibiofilm Activity of 3-Furancarboxaldehyde Increases the Virulence of Group A Streptococcus
Abstract
Background: Group A streptococcus (GAS, Streptococcus pyogenes), a multi-virulent, exclusive human pathogen responsible for various invasive and non-invasive diseases possesses biofilm forming phenomenon as one of its pathogenic armaments. Recently, antibiofilm agents have gained prime importance, since inhibiting the biofilm formation is expected to reduce development of antibiotic resistance and increase their susceptibility to the host immune cells.
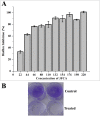
Principal findings: The current study demonstrates the antibiofilm activity of 3Furancarboxaldehyde (3FCA), a floral honey derived compound, against GAS biofilm, which was divulged using crystal violet assay, light microscopy, and confocal laser scanning microscopy. The report is extended to study its effect on various aspects of GAS (morphology, virulence, aggregation) at its minimal biofilm inhibitory concentration (132μg/ml). 3FCA was found to alter the growth pattern of GAS in solid and liquid medium and increased the rate of auto-aggregation. Electron microscopy unveiled the increase in extra polymeric substances around cell. Gene expression studies showed down-regulation of covR gene, which is speculated to be the prime target for the antibiofilm activity. Increased hyaluronic acid production and down regulation of srtB gene is attributed to the enhanced rate of auto-aggregation. The virulence genes (srv, mga, luxS and hasA) were also found to be over expressed, which was manifested with the increased susceptibility of the model organism Caenorhabditis elegans to 3FCA treated GAS. The toxicity of 3FCA was ruled out with no adverse effect on C. elegans.
Significance: Though 3FCA possess antibiofilm activity against GAS, it was also found to increase the virulence of GAS. This study demonstrates that, covR mediated antibiofilm activity may increase the virulence of GAS. This also emphasizes the importance to analyse the acclimatization response and virulence of the pathogen in the presence of antibiofilm compounds prior to their clinical trials.
Conflict of interest statement
Figures




References
-
- Kim S, Lee NY (2004) Epidemiology and antibiotic resistance of group A streptococci isolated from healthy school children in Korea. J Antimicrob Chemoth 54: 447–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

