A Workflow to Investigate Exposure and Pharmacokinetic Influences on High-Throughput in Vitro Chemical Screening Based on Adverse Outcome Pathways
- PMID: 25978103
- PMCID: PMC4710605
- DOI: 10.1289/ehp.1409450
A Workflow to Investigate Exposure and Pharmacokinetic Influences on High-Throughput in Vitro Chemical Screening Based on Adverse Outcome Pathways
Abstract
Background: Adverse outcome pathways (AOPs) link adverse effects in individuals or populations to a molecular initiating event (MIE) that can be quantified using in vitro methods. Practical application of AOPs in chemical-specific risk assessment requires incorporation of knowledge on exposure, along with absorption, distribution, metabolism, and excretion (ADME) properties of chemicals.
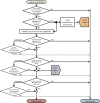
Objectives: We developed a conceptual workflow to examine exposure and ADME properties in relation to an MIE. The utility of this workflow was evaluated using a previously established AOP, acetylcholinesterase (AChE) inhibition.
Methods: Thirty chemicals found to inhibit human AChE in the ToxCast™ assay were examined with respect to their exposure, absorption potential, and ability to cross the blood-brain barrier (BBB). Structures of active chemicals were compared against structures of 1,029 inactive chemicals to detect possible parent compounds that might have active metabolites.
Results: Application of the workflow screened 10 "low-priority" chemicals of 30 active chemicals. Fifty-two of the 1,029 inactive chemicals exhibited a similarity threshold of ≥ 75% with their nearest active neighbors. Of these 52 compounds, 30 were excluded due to poor absorption or distribution. The remaining 22 compounds may inhibit AChE in vivo either directly or as a result of metabolic activation.
Conclusions: The incorporation of exposure and ADME properties into the conceptual workflow eliminated 10 "low-priority" chemicals that may otherwise have undergone additional, resource-consuming analyses. Our workflow also increased confidence in interpretation of in vitro results by identifying possible "false negatives."
Citation: Phillips MB, Leonard JA, Grulke CM, Chang DT, Edwards SW, Brooks R, Goldsmith MR, El-Masri H, Tan YM. 2016. A workflow to investigate exposure and pharmacokinetic influences on high-throughput in vitro chemical screening based on adverse outcome pathways. Environ Health Perspect 124:53-60; http://dx.doi.org/10.1289/ehp.1409450.
Conflict of interest statement
The U.S. EPA provided administrative review and approved this paper for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. EPA.
M.-R.G. and D.T.C. are employed by the Chemical Computing Group Inc., the publisher of the Molecular Operating Environment (MOE) software. The other authors declare they have no actual or potential competing financial interests.
Figures

References
-
- Abass K, Reponen P, Mattila S, Pelkonen O. Metabolism of carbosulfan II. Human interindividual variability in its in vitro hepatic biotransformation and the identification of the cytochrome p450 isoforms involved. Chem Biol Interact. 2010;185:163–173. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. - PubMed
-
- Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. - PubMed
-
- Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
