A microfibril assembly assay identifies different mechanisms of dominance underlying Marfan syndrome, stiff skin syndrome and acromelic dysplasias
- PMID: 25979247
- PMCID: PMC4492404
- DOI: 10.1093/hmg/ddv181
A microfibril assembly assay identifies different mechanisms of dominance underlying Marfan syndrome, stiff skin syndrome and acromelic dysplasias
Abstract
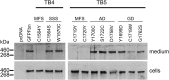
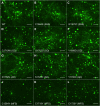
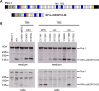
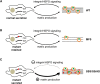
Fibrillin-1 is the major component of the 10-12 nm diameter extracellular matrix microfibrils. The majority of mutations affecting the human fibrillin-1 gene, FBN1, result in Marfan syndrome (MFS), a common connective tissue disorder characterised by tall stature, ocular and cardiovascular defects. Recently, stiff skin syndrome (SSS) and a group of syndromes known collectively as the acromelic dysplasias, which typically result in short stature, skin thickening and joint stiffness, have been linked to FBN1 mutations that affect specific domains of the fibrillin-1 protein. Despite their apparent phenotypic differences, dysregulation of transforming growth factor β (TGFβ) is a common factor in all of these disorders. Using a newly developed assay to track the secretion and incorporation of full-length, GFP-tagged fibrillin-1 into the extracellular matrix, we investigated whether or not there were differences in the secretion and microfibril assembly profiles of fibrillin-1 variants containing substitutions associated with MFS, SSS or the acromelic dysplasias. We show that substitutions in fibrillin-1 domains TB4 and TB5 that cause SSS and the acromelic dysplasias do not prevent fibrillin-1 from being secreted or assembled into microfibrils, whereas MFS-associated substitutions in these domains result in a loss of recombinant protein in the culture medium and no association with microfibrils. These results suggest fundamental differences in the dominant pathogenic mechanisms underlying MFS, SSS and the acromelic dysplasias, which give rise to TGFβ dysregulation associated with these diseases.
© The Author 2015. Published by Oxford University Press.
Figures

References
-
- Jones C.J., Sear C.H., Grant M.E. (1980) An ultrastructural study of fibroblasts derived from bovine ligamentum nuchae and their capacity for elastogenesis in culture. J. Pathol., 131, 35–53. - PubMed
-
- Kewley M.A., Williams G., Steven F.S. (1978) Studies of elastic tissue formation in the developing bovine ligamentum nuchae. J. Pathol., 124, 95–101. - PubMed
-
- Bax D.V., Bernard S.E., Lomas A., Morgan A., Humphries J., Shuttleworth C.A., Humphries M.J., Kielty C.M. (2003) Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J. Biol. Chem., 278, 34605–34616. - PubMed
-
- Jovanovic J., Takagi J., Choulier L., Abrescia N.G., Stuart D.I., van der Merwe P.A., Mardon H.J., Handford P.A. (2007) alphaVbeta6 is a novel receptor for human fibrillin-1. Comparative studies of molecular determinants underlying integrin-rgd affinity and specificity. J. Biol. Chem., 282, 6743–6751. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

