Cancer cell surface induced peptide folding allows intracellular translocation of drug
- PMID: 25979324
- PMCID: PMC4458427
- DOI: 10.1016/j.jconrel.2015.05.267
Cancer cell surface induced peptide folding allows intracellular translocation of drug
Abstract
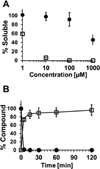
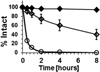
Many lead molecules identified in drug discovery campaigns are eliminated from consideration due to poor solubility and low cell permeability. These orphaned molecules could have clinical value if solubilized and delivered properly. SVS-1 is a de novo designed peptide that preferentially folds at the surface of tumor cells, adopting a β-hairpin conformation that rapidly translocates into the cytoplasm, and ultimately nucleus, of cells. SVS-1 is stable in serum and small molecules attached to the peptide are effectively delivered to cancer cells via mechanisms involving physical translocation and, to a lesser extent, clathrin-dependent endocytosis. For example, ligating the model hydrophobic drug Paclitaxel (PTX) to SVS-1 improved its aqueous solubility by ~1000-fold and successfully delivered and released PTX to cancer cells in vitro and tumors in vivo without toxic adjuvants. These results suggest that SVS-1 can serve as a simple, effective delivery platform for molecules with poor solubility and permeability.
Keywords: Biodistribution; Cell-penetrating peptide; Drug delivery; In vivo efficacy; Paclitaxel; Translocation.
Published by Elsevier B.V.
Figures






References
-
- Geisow MJ. Fluorescein conjugates as indicators of subcellular pH: A critical evaluation. Exp. Cell Res. 1984;150:29–35. - PubMed
-
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the Internalization Mechanism of Cationic Cell-penetrating Peptides. J. Biol. Chem. 2003;278:31192–31201. - PubMed
-
- Massodi I, Bidwell Iii GL, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J. Controlled Release. 2005;108:396–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

