Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension
- PMID: 25979327
- PMCID: PMC4477514
- DOI: 10.1016/j.jconrel.2015.05.261
Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension
Abstract
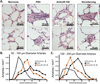
Therapies that exploit RNA interference (RNAi) hold great potential for improving disease outcomes. However, there are several challenges that limit the application of RNAi therapeutics. One of the most important challenges is effective delivery of oligonucleotides to target cells and reduced delivery to non-target cells. We have previously developed a functionalized cationic lipopolyamine (Star:Star-mPEG-550) for in vivo delivery of siRNA to pulmonary vascular cells. This optimized lipid formulation enhances the retention of siRNA in mouse lungs and achieves significant knockdown of target gene expression for at least 10days following a single intravenous injection. Although this suggests great potential for developing lung-directed RNAi-based therapies, the application of Star:Star-mPEG mediated delivery of RNAi based therapies for pulmonary vascular diseases such as pulmonary arterial hypertension (PAH) remains unknown. We identified differential expression of several microRNAs known to regulate cell proliferation, cell survival and cell fate that are associated with development of PAH, including increased expression of microRNA-145 (miR-145). Here we test the hypothesis that Star:Star-mPEG mediated delivery of an antisense oligonucleotide against miR-145 (antimiR-145) will improve established PAH in rats. We performed a series of experiments testing the in vivo distribution, toxicity, and efficacy of Star:Star-mPEG mediated delivery of antimiR-145 in rats with Sugen-5416/hypoxia induced PAH. We showed that after subchronic therapy of three intravenous injections over 5weeks at 2mg/kg, antimiR-145 accumulated in rat lung tissue and reduced expression of endogenous miR-145. Using a novel in situ hybridization approach, we demonstrated substantial distribution of antimiR-145 in the lungs as well as the liver, kidney, and spleen. We assessed toxic effects of Star:Star-mPEG/antimiR-145 with serial complete blood counts of leukocytes and serum metabolic panels, gross pathology, and histopathology and did not detect significant off-target effects. AntimiR-145 reduced the degree of pulmonary arteriopathy, reduced the severity of pulmonary hypertension, and reduced the degree of cardiac dysfunction. The results establish effective and low toxicity of lung delivery of a miRNA-145 inhibitor using functionalized cationic lipopolyamine nanoparticles to repair pulmonary arteriopathy and improve cardiac function in rats with severe PAH.
Keywords: Antisense oligonucleotide; Lipid nanoparticle; Lung delivery; MicroRNA-145; Pulmonary hypertension; Sugen5416/hypoxia.
Copyright © 2015. Published by Elsevier B.V.
Figures





Similar articles
-
Lipid Nanoparticles Composed of Quaternary Amine-Tertiary Amine Cationic Lipid Combination (QTsome) for Therapeutic Delivery of AntimiR-21 for Lung Cancer.Mol Pharm. 2016 Feb 1;13(2):653-62. doi: 10.1021/acs.molpharmaceut.5b00878. Epub 2016 Jan 19. Mol Pharm. 2016. PMID: 26741162
-
Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension.Am J Respir Crit Care Med. 2012 Feb 15;185(4):409-19. doi: 10.1164/rccm.201106-1093OC. Epub 2011 Dec 8. Am J Respir Crit Care Med. 2012. PMID: 22161164
-
Reversal of MicroRNA Dysregulation in an Animal Model of Pulmonary Hypertension.PLoS One. 2016 Jan 27;11(1):e0147827. doi: 10.1371/journal.pone.0147827. eCollection 2016. PLoS One. 2016. PMID: 26815432 Free PMC article.
-
Novel therapeutic approaches for pulmonary arterial hypertension: Unique molecular targets to site-specific drug delivery.J Control Release. 2015 Aug 10;211:118-33. doi: 10.1016/j.jconrel.2015.05.287. Epub 2015 May 31. J Control Release. 2015. PMID: 26036906 Review.
-
The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt A):129-137. doi: 10.1016/j.addr.2016.01.022. Epub 2016 Feb 18. Adv Drug Deliv Rev. 2016. PMID: 26900977 Review.
Cited by
-
Current Overview of the Biology and Pharmacology in Sugen/Hypoxia-Induced Pulmonary Hypertension in Rats.J Aerosol Med Pulm Drug Deliv. 2024 Oct;37(5):241-283. doi: 10.1089/jamp.2024.0016. J Aerosol Med Pulm Drug Deliv. 2024. PMID: 39388691 Review.
-
NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension.Sci Transl Med. 2018 Jun 13;10(445):eaap7294. doi: 10.1126/scitranslmed.aap7294. Sci Transl Med. 2018. PMID: 29899023 Free PMC article.
-
Silencing a Multifunctional microRNA Is Beneficial for Stroke Recovery.Front Mol Neurosci. 2018 Feb 23;11:58. doi: 10.3389/fnmol.2018.00058. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29527155 Free PMC article. Review.
-
CircRNA-based AntimiR therapy: A novel approach to hypertension treatment.Noncoding RNA Res. 2025 May 5;13:94-108. doi: 10.1016/j.ncrna.2025.05.001. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40487299 Free PMC article. Review.
-
Targeting Inflammasome Activation in COVID-19: Delivery of RNA Interference-Based Therapeutic Molecules.Biomedicines. 2021 Dec 3;9(12):1823. doi: 10.3390/biomedicines9121823. Biomedicines. 2021. PMID: 34944639 Free PMC article. Review.
References
-
- Voelkel NF, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. - PubMed
-
- Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43(12) Suppl S:13S–24S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

