Superantigens produced by catheter-associated Staphylococcus aureus elicit systemic inflammatory disease in the absence of bacteremia
- PMID: 25979434
- PMCID: PMC4501677
- DOI: 10.1189/jlb.4A1214-577RR
Superantigens produced by catheter-associated Staphylococcus aureus elicit systemic inflammatory disease in the absence of bacteremia
Abstract
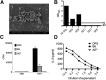
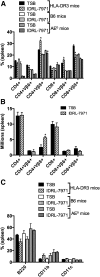
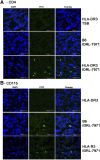
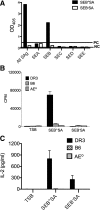
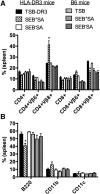
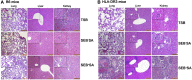
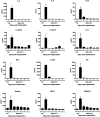
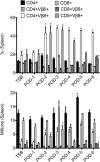
SAgs, produced by Staphylococcus aureus, play a major role in the pathogenesis of invasive staphylococcal diseases by inducing potent activation of the immune system. However, the role of SAgs, produced by S. aureus, associated with indwelling devices or tissues, are not known. Given the prevalence of device-associated infection with toxigenic S. aureus in clinical settings and the potency of SAgs, we hypothesized that continuous exposure to SAgs produced by catheter-associated S. aureus could have systemic consequences. To investigate these effects, we established a murine in vivo catheter colonization model. One centimeter long intravenous catheters were colonized with a clinical S. aureus isolate producing SAgs or isogenic S. aureus strains, capable or incapable of producing SAg. Catheters were subcutaneously implanted in age-matched HLA-DR3, B6, and AE(o) mice lacking MHC class II molecules and euthanized 7 d later. There was no evidence of systemic infection. However, in HLA-DR3 transgenic mice, which respond robustly to SSAgs, the SSAg-producing, but not the nonproducing strains, caused a transient increase in serum cytokine levels and a protracted expansion of splenic CD4(+) T cells expressing SSAg-reactive TCR Vβ8. Lungs, livers, and kidneys from these mice showed infiltration with CD4(+) and CD11b(+) cells. These findings were absent in B6 and AE(o) mice, which are known to respond poorly to SSAgs. Overall, our novel findings suggest that systemic immune activation elicited by SAgs, produced by S. aureus colonizing foreign bodies, could have clinical consequences in humans.
Keywords: HLA class II; T lymphocytes; cytokines; immunopathology; transgenic mice.
© Society for Leukocyte Biology.
Figures


References
-
- O’Grady N. P., Alexander M., Burns L. A., Dellinger E. P., Garland J., Heard S. O., Lipsett P. A., Masur H., Mermel L. A., Pearson M. L., Raad I. I., Randolph A. G., Rupp M. E., Saint S.; Healthcare Infection Control Practices Advisory Committee (2011) Guidelines for the prevention of intravascular catheter-related infections. Am. J. Infect. Control 39 (4 Suppl 1) S1–S34. - PubMed
-
- Hidron A. I., Edwards J. R., Patel J., Horan T. C., Sievert D. M., Pollock D. A., Fridkin S. K.; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities (2008) NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29, 996–1011. - PubMed
-
- Brady R. A., Leid J. G., Calhoun J. H., Costerton J. W., Shirtliff M. E. (2008) Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52, 13–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

