Asymmetric cell division of granule neuron progenitors in the external granule layer of the mouse cerebellum
- PMID: 25979710
- PMCID: PMC4571082
- DOI: 10.1242/bio.009886
Asymmetric cell division of granule neuron progenitors in the external granule layer of the mouse cerebellum
Abstract
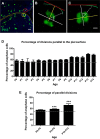
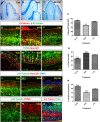
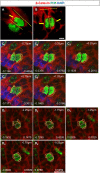
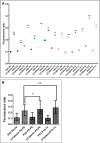
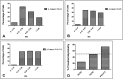
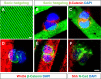
The plane of division of granule neuron progenitors (GNPs) was analysed with respect to the pial surface in P0 to P14 cerebellum and the results showed that there was a significant bias towards the plane of cell division being parallel to pial surface across this developmental window. In addition, the distribution of β-Catenin in anaphase cells was analysed, which showed that there was a significant asymmetry in the distribution of β-Catenin in dividing GNPs. Further, inhibition of Sonic Hedgehog (Shh) signalling had an effect on plane of cell division. Asymmetric distribution of β-Catenin was shown to occur towards the source of a localized extracellular cue.
Keywords: Cell division; Cerebellum; Sonic hedgehog; β-Catenin.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors certify that there is no conflict of interest with any organization regarding the material discussed in the manuscript.
Figures
References
-
- Anne S. L., Govek E. E., Ayrault O., Kim J. H., Zhu X., Murphy D. A., Van Aelst L., Roussel M. F. and Hatten M. E. (2013). WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation. PLoS ONE 8, e81769 10.1371/journal.pone.0081769 - DOI - PMC - PubMed
-
- Attardo A., Calegari F., Haubensak W., Wilsch-Bräuninger M. and Huttner W. B. (2008). Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS ONE 3, e2388 10.1371/journal.pone.0002388 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

