Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells
- PMID: 25979873
- PMCID: PMC4506263
- DOI: 10.1158/0008-5472.CAN-14-3041
Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells
Abstract
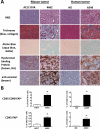
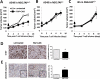
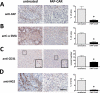
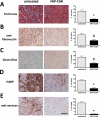
Malignant cells drive the generation of a desmoplastic and immunosuppressive tumor microenvironment. Cancer-associated stromal cells (CASC) are a heterogeneous population that provides both negative and positive signals for tumor cell growth and metastasis. Fibroblast activation protein (FAP) is a marker of a major subset of CASCs in virtually all carcinomas. Clinically, FAP expression serves as an independent negative prognostic factor for multiple types of human malignancies. Prior studies established that depletion of FAP(+) cells inhibits tumor growth by augmenting antitumor immunity. However, the potential for immune-independent effects on tumor growth have not been defined. Herein, we demonstrate that FAP(+) CASCs are required for maintenance of the provisional tumor stroma because depletion of these cells, by adoptive transfer of FAP-targeted chimeric antigen receptor (CAR) T cells, reduced extracellular matrix proteins and glycosaminoglycans. Adoptive transfer of FAP-CAR T cells also decreased tumor vascular density and restrained growth of desmoplastic human lung cancer xenografts and syngeneic murine pancreatic cancers in an immune-independent fashion. Adoptive transfer of FAP-CAR T cells also restrained autochthonous pancreatic cancer growth. These data distinguish the function of FAP(+) CASCs from other CASC subsets and provide support for further development of FAP(+) stromal cell-targeted therapies for the treatment of solid tumors.
©2015 American Association for Cancer Research.
Figures



References
-
- Jacob M, Chang L, Pure E. Fibroblast activation protein in remodeling tissues. Curr Mol Med. 2012;12:1220–43. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

