Molybdenum Availability Is Key to Nitrate Removal in Contaminated Groundwater Environments
- PMID: 25979890
- PMCID: PMC4495186
- DOI: 10.1128/AEM.00917-15
Molybdenum Availability Is Key to Nitrate Removal in Contaminated Groundwater Environments
Abstract
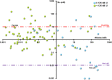
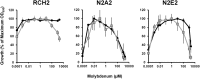
The concentrations of molybdenum (Mo) and 25 other metals were measured in groundwater samples from 80 wells on the Oak Ridge Reservation (ORR) (Oak Ridge, TN), many of which are contaminated with nitrate, as well as uranium and various other metals. The concentrations of nitrate and uranium were in the ranges of 0.1 μM to 230 mM and <0.2 nM to 580 μM, respectively. Almost all metals examined had significantly greater median concentrations in a subset of wells that were highly contaminated with uranium (≥126 nM). They included cadmium, manganese, and cobalt, which were 1,300- to 2,700-fold higher. A notable exception, however, was Mo, which had a lower median concentration in the uranium-contaminated wells. This is significant, because Mo is essential in the dissimilatory nitrate reduction branch of the global nitrogen cycle. It is required at the catalytic site of nitrate reductase, the enzyme that reduces nitrate to nitrite. Moreover, more than 85% of the groundwater samples contained less than 10 nM Mo, whereas concentrations of 10 to 100 nM Mo were required for efficient growth by nitrate reduction for two Pseudomonas strains isolated from ORR wells and by a model denitrifier, Pseudomonas stutzeri RCH2. Higher concentrations of Mo tended to inhibit the growth of these strains due to the accumulation of toxic concentrations of nitrite, and this effect was exacerbated at high nitrate concentrations. The relevance of these results to a Mo-based nitrate removal strategy and the potential community-driving role that Mo plays in contaminated environments are discussed.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Riley RG, Zachara J. 1992. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. Pacific Northwest Laboratory, Richland, WA.
-
- Brooks SC. 2001. Waste characteristics of the former S-3 ponds and outline of uranium chemistry relevant to NABIR Field Research Center studies. NABIR Field Research Center, Oak Ridge, TN.
-
- Revil A, Skold M, Karaoulis M, Schmutz M, Hubbard S, Mehlhorn T, Watson D. 2013. Hydrogeophysical investigations of the former S-3 ponds contaminant plumes, Oak Ridge Integrated Field Research Challenge site, Tennessee. Geophysics 78:EN29–EN41.
-
- Waldron PJ, Wu L, Nostrand JDV, Schadt CW, He Z, Watson DB, Jardine PM, Palumbo AV, Hazen TC, Zhou J. 2009. Functional gene array-based analysis of microbial community structure in groundwaters with a gradient of contaminant levels. Environ Sci Technol 43:3529–3534. doi:10.1021/es803423p. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

