Genetics and Physiology of Acetate Metabolism by the Pta-Ack Pathway of Streptococcus mutans
- PMID: 25979891
- PMCID: PMC4495203
- DOI: 10.1128/AEM.01160-15
Genetics and Physiology of Acetate Metabolism by the Pta-Ack Pathway of Streptococcus mutans
Abstract
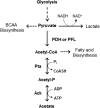
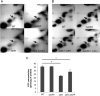
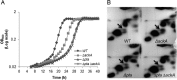
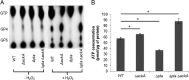
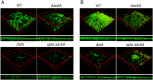
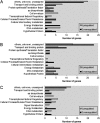
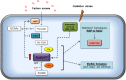
In the dental caries pathogen Streptococcus mutans, phosphotransacetylase (Pta) catalyzes the conversion of acetyl coenzyme A (acetyl-CoA) to acetyl phosphate (AcP), which can be converted to acetate by acetate kinase (Ack), with the concomitant generation of ATP. A ΔackA mutant displayed enhanced accumulation of AcP under aerobic conditions, whereas little or no AcP was observed in the Δpta or Δpta ΔackA mutant. The Δpta and Δpta ΔackA mutants also had diminished ATP pools compared to the size of the ATP pool for the parental or ΔackA strain. Surprisingly, when exposed to oxidative stress, the Δpta ΔackA strain appeared to regain the capacity to produce AcP, with a concurrent increase in the size of the ATP pool compared to that for the parental strain. The ΔackA and Δpta ΔackA mutants exhibited enhanced (p)ppGpp accumulation, whereas the strain lacking Pta produced less (p)ppGpp than the wild-type strain. The ΔackA and Δpta ΔackA mutants displayed global changes in gene expression, as assessed by microarrays. All strains lacking Pta, which had defects in AcP production under aerobic conditions, were impaired in their abilities to form biofilms when glucose was the growth carbohydrate. Collectively, these data demonstrate the complex regulation of the Pta-Ack pathway and critical roles for these enzymes in processes that appear to be essential for the persistence and pathogenesis of S. mutans.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
CcpA and CodY Coordinate Acetate Metabolism in Streptococcus mutans.Appl Environ Microbiol. 2017 Mar 17;83(7):e03274-16. doi: 10.1128/AEM.03274-16. Print 2017 Apr 1. Appl Environ Microbiol. 2017. PMID: 28130304 Free PMC article.
-
Instability of ackA (acetate kinase) mutations and their effects on acetyl phosphate and ATP amounts in Streptococcus pneumoniae D39.J Bacteriol. 2010 Dec;192(24):6390-400. doi: 10.1128/JB.00995-10. Epub 2010 Oct 15. J Bacteriol. 2010. PMID: 20952579 Free PMC article.
-
Pathway identification combining metabolic flux and functional genomics analyses: acetate and propionate activation by Corynebacterium glutamicum.J Biotechnol. 2009 Mar 10;140(1-2):75-83. doi: 10.1016/j.jbiotec.2008.12.014. Epub 2008 Dec 27. J Biotechnol. 2009. PMID: 19162097
-
Acetate kinase: not just a bacterial enzyme.Trends Microbiol. 2006 Jun;14(6):249-53. doi: 10.1016/j.tim.2006.04.001. Epub 2006 May 4. Trends Microbiol. 2006. PMID: 16678422 Review.
-
Tracking acetate through a journey of living world: Evolution as alternative cellular fuel with potential for application in cancer therapeutics.Life Sci. 2018 Dec 15;215:86-95. doi: 10.1016/j.lfs.2018.11.004. Epub 2018 Nov 5. Life Sci. 2018. PMID: 30408472 Review.
Cited by
-
Urethane Dimethacrylate Influences the Cariogenic Properties of Streptococcus Mutans.Materials (Basel). 2021 Feb 21;14(4):1015. doi: 10.3390/ma14041015. Materials (Basel). 2021. PMID: 33669956 Free PMC article.
-
Cariogenic potential of sweet flavors in electronic-cigarette liquids.PLoS One. 2018 Sep 7;13(9):e0203717. doi: 10.1371/journal.pone.0203717. eCollection 2018. PLoS One. 2018. PMID: 30192874 Free PMC article.
-
Metagenome-assembled genomes provide insight into the metabolic potential during early production of Hydraulic Fracturing Test Site 2 in the Delaware Basin.Front Microbiol. 2024 Jun 12;15:1376536. doi: 10.3389/fmicb.2024.1376536. eCollection 2024. Front Microbiol. 2024. PMID: 38933028 Free PMC article.
-
New Microbial Lineages Capable of Carbon Fixation and Nutrient Cycling in Deep-Sea Sediments of the Northern South China Sea.Appl Environ Microbiol. 2019 Jul 18;85(15):e00523-19. doi: 10.1128/AEM.00523-19. Print 2019 Aug 1. Appl Environ Microbiol. 2019. PMID: 31126943 Free PMC article.
-
RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms.Front Microbiol. 2017 Jun 8;8:1036. doi: 10.3389/fmicb.2017.01036. eCollection 2017. Front Microbiol. 2017. PMID: 28642749 Free PMC article.
References
-
- Lemos JA, Abranches J, Burne RA. 2005. Responses of cariogenic streptococci to environmental stresses. Curr Issues Mol Biol 7:95–107. - PubMed
-
- Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439. doi:10.1073/pnas.172501299. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

