Lung cancer in the era of precision medicine
- PMID: 25979927
- PMCID: PMC4505624
- DOI: 10.1158/1078-0432.CCR-14-2748
Lung cancer in the era of precision medicine
Abstract
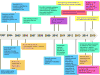
The past decade has been transformative for lung cancer patients, physicians, and scientists. The discovery of EGFR mutations that confer sensitivity to tyrosine kinase inhibitors in lung adenocarcinomas in 2004 heralded the beginning of the era of precision medicine for lung cancer. Indeed, it precipitated concerted efforts by many investigators to define molecular subgroups of lung cancer, characterize the genomic landscape of lung cancer subtypes, identify novel therapeutic targets, and define mechanisms of sensitivity and resistance to targeted therapies. The fruits of these efforts are visible every day now in lung cancer clinics: Patients receive molecular testing to determine whether their tumor harbors an actionable mutation, new and improved targeted therapies that can overcome resistance to first-generation drugs are in clinical trials, and drugs targeting the immune system are showing activity in patients. This extraordinary promise is tempered by the sobering fact that even the newest treatments for metastatic disease are rarely curative and are effective only in a small fraction of all patients. Ongoing and future efforts to find new vulnerabilities of lung cancers, unravel the complexity of drug resistance, increase the efficacy of immunotherapies, and perform biomarker-driven clinical trials are necessary to improve outcomes for patients with lung cancer.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- American Cancer Society. Cancer facts & figures 2015 [PDF on the Internet] Atlanta (GA): American Cancer Society; 2015. [cited 2015 Mar 27]. Report No.: 500815. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/a....
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. - PubMed
-
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Engl J Med. 2004;350:2129–39. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

