Endoplasmic reticulum and oxidant stress mediate nuclear factor-κB activation in the subfornical organ during angiotensin II hypertension
- PMID: 25980014
- PMCID: PMC4587629
- DOI: 10.1152/ajpcell.00223.2014
Endoplasmic reticulum and oxidant stress mediate nuclear factor-κB activation in the subfornical organ during angiotensin II hypertension
Abstract
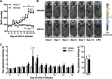
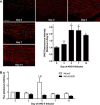
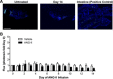
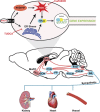
Endoplasmic reticulum (ER) stress and reactive oxygen species (ROS) generation in the brain circumventricular subfornical organ (SFO) mediate the central hypertensive actions of Angiotensin II (ANG II). However, the downstream signaling events remain unclear. Here we tested the hypothesis that angiotensin type 1a receptors (AT1aR), ER stress, and ROS induce activation of the transcription factor nuclear factor-κB (NF-κB) during ANG II-dependent hypertension. To spatiotemporally track NF-κB activity in the SFO throughout the development of ANG II-dependent hypertension, we used SFO-targeted adenoviral delivery and longitudinal bioluminescence imaging in mice. During low-dose infusion of ANG II, bioluminescence imaging revealed a prehypertensive surge in NF-κB activity in the SFO at a time point prior to a significant rise in arterial blood pressure. SFO-targeted ablation of AT1aR, inhibition of ER stress, or adenoviral scavenging of ROS in the SFO prevented the ANG II-induced increase in SFO NF-κB. These findings highlight the utility of bioluminescence imaging to longitudinally track transcription factor activation during the development of ANG II-dependent hypertension and reveal an AT1aR-, ER stress-, and ROS-dependent prehypertensive surge in NF-κB activity in the SFO. Furthermore, the increase in NF-κB activity before a rise in arterial blood pressure suggests a causal role for SFO NF-κB in the development of ANG II-dependent hypertension.
Keywords: NF-κB, ER stress; angiotensin II; central nervous system; hypertension.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Blume A, Herdegen T, Unger T. Angiotensin peptides and inducible transcription factors. J Mol Med 77: 339–357, 1999. - PubMed
-
- Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kB (NF-kB) transcription factor. 212: 155–169, 2000. - PubMed
-
- Butz GM, Davisson RL. Chronic blood pressure recording in pregnant mice by radiotelemetry. Physiol Genomics 2001.
-
- Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 59: 869–876, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

