XB130 translocation to microfilamentous structures mediates NNK-induced migration of human bronchial epithelial cells
- PMID: 25980441
- PMCID: PMC4627235
- DOI: 10.18632/oncotarget.3777
XB130 translocation to microfilamentous structures mediates NNK-induced migration of human bronchial epithelial cells
Abstract
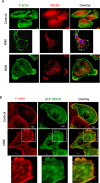
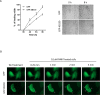
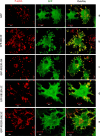
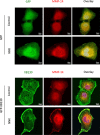
Cigarette smoking contributes to the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nicotine-derived nitrosamine ketone (NNK) is the most potent carcinogen among cigarette smoking components, and is known to enhance migration of cancer cells. However, the effect of NNK on normal human bronchial epithelial cells is not well studied. XB130 is a member of actin filament associated protein family and is involved in cell morphology changes, cytoskeletal rearrangement and outgrowth formation, as well as cell migration. We hypothesized that XB130 mediates NNK-induced migration of normal human bronchial epithelial cells. Our results showed that, after NNK stimulation, XB130 was translocated to the cell periphery and enriched in cell motility-associated structures, such as lamellipodia, in normal human bronchial epithelial BEAS2B cells. Moreover, overexpression of XB130 significantly enhanced NNK-induced migration, which requires both the N- and C-termini of XB130. Overexpression of XB130 enhanced NNK-induced protein tyrosine phosphorylation and promoted matrix metalloproteinase-14 translocation to cell motility-associated cellular structures after NNK stimulation. XB130-mediated NNK-induced cell migration may contribute to airway epithelial repair; however, it may also be involved in cigarette smoking-related chronic obstructive pulmonary disease and lung cancer.
Keywords: F-actin association; airway epithelial cell migration; cortactin; intracellular signal transduction.
Figures




References
-
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
-
- West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

