Modulation of Igβ is essential for the B cell selection in germinal center
- PMID: 25980548
- PMCID: PMC4650814
- DOI: 10.1038/srep10303
Modulation of Igβ is essential for the B cell selection in germinal center
Erratum in
-
Erratum: Modulation of Igβ is essential for the B cell selection in germinal center.Sci Rep. 2015 Aug 5;5:12208. doi: 10.1038/srep12208. Sci Rep. 2015. PMID: 26244941 Free PMC article. No abstract available.
Abstract
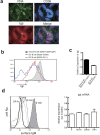
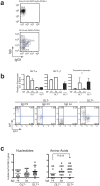
The positive and negative selection of antigen-reactive B cells take place in the germinal center (GC) during an immune responses. However, the precise molecular mechanisms underlying these selection machineries, including the involvement of antigen receptor signaling molecules, remain to be elucidated. We found that expression levels of Igα and Igβ, which are the essential components of B cell antigen-receptor complex, were differentially regulated in GC B cells and that the expression of Igβ was more prominently down-regulated in a portion of GC B cells. The suppression of Igβ down-regulation reduced the number of GL7(+)GC B cells and the affinity maturation in T-dependent responses was markedly impaired. In addition, the disease phenotypes in autoimmune-prone mice were ameliorated by blocking of Igβ down-regulation. These results suggest that Igβ down-regulation is involved in the normal positive selection in GC and the accumulation of autoreactive B cells in autoimmune-prone mice.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

