Changes in energy during treatment of depression: an analysis of duloxetine in double-blind placebo-controlled trials
- PMID: 25980552
- PMCID: PMC4682452
- DOI: 10.1111/ijcp.12658
Changes in energy during treatment of depression: an analysis of duloxetine in double-blind placebo-controlled trials
Abstract
Aims: The aim of this study was to assess how quickly and effectively duloxetine improves energy compared with placebo in patients with major depressive disorder (MDD).
Methods: Data from 10 randomised, double-blind, placebo-controlled clinical trials examining duloxetine (40-60 mg/day) vs. placebo in patients diagnosed with MDD were analysed. Change from baseline at Week 1 through Week 8 in Hamilton Depression Rating Scale (HAM-D) retardation subscale score (Item 1 - depressed mood, Item 7 - work and activities, Item 8 - retardation and Item 14 - genital symptoms) was assessed with mixed model repeated measures analysis. Positive predictive values and negative predictive values were calculated for predictor analysis.
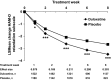
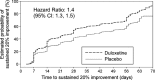
Results: Patients treated with duloxetine (N = 1522) experienced statistically significantly (p ≤ 0.05) greater reductions in HAM-D retardation subscale scores vs. placebo (N = 1180) starting at Week 1 throughout Week 8 of treatment. Of the patients with early energy improvement (≥ 20% reduction in HAM-D retardation subscale scores) at Week 1, 48% achieved remission (HAM-D total score ≤ 7) at Week 8; 48% and 46% of patients who experienced early energy improvement at Weeks 2 and 4, respectively, achieved remission at Week 8.
Discussion: We demonstrated that treatment with duloxetine, quickly and with increasing magnitude over treatment time, improves low energy symptoms. As early as 1 week after starting treatment with duloxetine, improvement of low energy may serve as a predictor of remission at end-point.
Conclusions: Treatment with duloxetine improves energy in patients with MDD and early response in retardation may serve as a modest predictor of remission at end-point.
Clinical trials registration: ClinicalTrials.gov. Study Identifiers: NCT00036335; NCT00073411; NCT00406848 and NCT00536471. Studies HMAQa, HMAQb, HMATa, HMATb, HMBHa and HMBHb predate the registration requirement.
Data posting: ClinicalTrials.gov. Study Identifiers: NCT00406848; NCT00536471.
© 2015 Eli Lilly Japan K.K. International Journal of Clinical Practice Published by John Wiley & Sons Ltd.
Figures


References
-
- Joliat MJ, Brown EB, Miner CM. Changes in energy after switching from daily citalopram, paroxetine, or sertraline to once-weekly fluoxetine. J Clin Psychopharmacol. 2004;24:464–7. - PubMed
-
- Judge R, Plewes JM, Kumar V, Koke SC, Kopp JB. Changes in energy during treatment of depression: an analysis of fluoxetine in double-blind, placebo-controlled trials. J Clin Psychopharmacol. 2000;20:666–72. - PubMed
-
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14:139–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
