Prescriber and patient-oriented behavioural interventions to improve use of malaria rapid diagnostic tests in Tanzania: facility-based cluster randomised trial
- PMID: 25980737
- PMCID: PMC4445498
- DOI: 10.1186/s12916-015-0346-z
Prescriber and patient-oriented behavioural interventions to improve use of malaria rapid diagnostic tests in Tanzania: facility-based cluster randomised trial
Abstract
Background: The increasing investment in malaria rapid diagnostic tests (RDTs) to differentiate malarial and non-malarial fevers, and an awareness of the need to improve case management of non-malarial fever, indicates an urgent need for high quality evidence on how best to improve prescribers' practices.
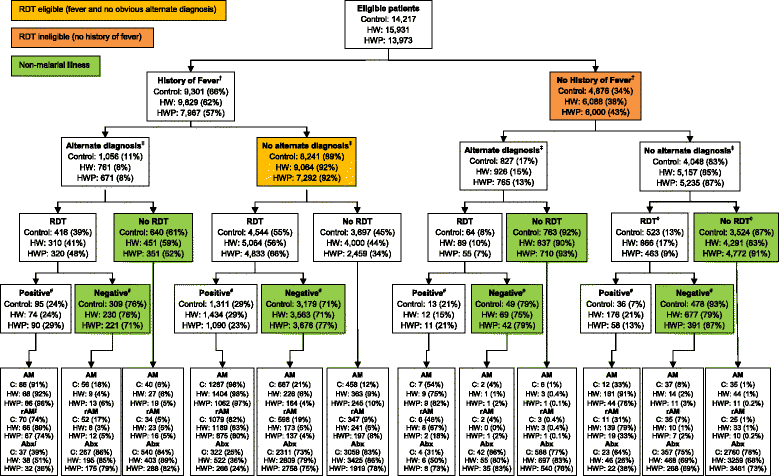
Methods: A three-arm stratified cluster-randomised trial was conducted in 36 primary healthcare facilities from September 2010 to March 2012 within two rural districts in northeast Tanzania where malaria transmission has been declining. Interventions were guided by formative mixed-methods research and were introduced in phases. Prescribing staff from all facilities received standard Ministry of Health RDT training. Prescribers from facilities in the health worker (HW) and health worker-patient (HWP) arms further participated in small interactive peer-group training sessions with the HWP additionally receiving clinic posters and patient leaflets. Performance feedback and motivational mobile-phone text messaging (SMS) were added to the HW and HWP arms in later phases. The primary outcome was the proportion of patients with a non-severe, non-malarial illness incorrectly prescribed a (recommended) antimalarial. Secondary outcomes investigated RDT uptake, adherence to results, and antibiotic prescribing.
Results: Standard RDT training reduced pre-trial levels of antimalarial prescribing, which was sustained throughout the trial. Both interventions significantly lowered incorrect prescribing of recommended antimalarials from 8% (749/8,942) in the standard training arm to 2% (250/10,118) in the HW arm (adjusted RD (aRD) 4%; 95% confidence interval (CI) 1% to 6%; P = 0.008) and 2% (184/10,163) in the HWP arm (aRD 4%; 95% CI 1% to 6%; P = 0.005). Small group training and SMS were incrementally effective. There was also a significant reduction in the prescribing of antimalarials to RDT-negatives but no effect on RDT-positives receiving an ACT. Antibiotic prescribing was significantly lower in the HWP arm but had increased in all arms compared with pre-trial levels.
Conclusions: Small group training with SMS was associated with an incremental and sustained improvement in prescriber adherence to RDT results and reducing over-prescribing of antimalarials to close to zero. These interventions may become increasingly important to cope with the wider range of diagnostic and treatment options for patients with acute febrile illness in Africa.
Trial registration: ClinicalTrials.gov NCT01292707.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

