One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial
- PMID: 25980900
- PMCID: PMC4744775
- DOI: 10.1111/dom.12498
One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial
Abstract
Aims: To confirm, in a 26-week extension study, the sustained efficacy and safety of a fixed combination of insulin degludec and liraglutide (IDegLira) compared with either insulin degludec or liraglutide alone, in patients with type 2 diabetes.
Methods: Insulin-naïve adults with type 2 diabetes randomized to once-daily IDegLira, insulin degludec or liraglutide, in addition to metformin ± pioglitazone, continued their allocated treatment in this preplanned 26-week extension of the DUAL I trial.
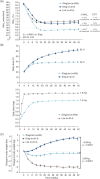
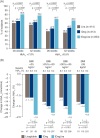
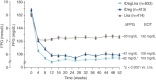
Results: A total of 78.8% of patients (1311/1663) continued into the extension phase. The mean glycated haemoglobin (HbA1c) concentration at 52 weeks was reduced from baseline by 1.84% (20.2 mmol/mol) for the IDegLira group, 1.40% (15.3 mmol/mol) for the insulin degludec group and 1.21% (13.2 mmol/mol) for the liraglutide group. Of the patients on IDegLira, 78% achieved an HbA1c of <7% (53 mmol/mol) versus 63% of the patients on insulin degludec and 57% of those on liraglutide. The mean fasting plasma glucose concentration at the end of the trial was similar for IDegLira (5.7 mmol/l) and insulin degludec (6.0 mmol/l), but higher for liraglutide (7.3 mmol/l). At 52 weeks, the daily insulin dose was 37% lower with IDegLira (39 units) than with insulin degludec (62 units). IDegLira was associated with a significantly greater decrease in body weight (estimated treatment difference, -2.80 kg, p < 0.0001) and a 37% lower rate of hypoglycaemia compared with insulin degludec. Overall, all treatments were well tolerated and no new adverse events or tolerability issues were observed for IDegLira.
Conclusions: These 12-month data, derived from a 26-week extension of the DUAL I trial, confirm the initial 26-week main phase results and the sustainability of the benefits of IDegLira compared with its components in glycaemic efficacy, safety and tolerability.
Keywords: diabetes therapy; hypoglycaemia; insulin degludec; liraglutide.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet 2014; 384: 2228–2234. - PubMed
-
- Goldenberg R. Insulin plus incretin agent combination therapy in type 2 diabetes: a systematic review. Curr Med Res Opin 2014; 30: 431–445. - PubMed
-
- Gough SC, Bode B, Woo V et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 885–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

