Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis
- PMID: 25981038
- PMCID: PMC4449334
- DOI: 10.1016/j.celrep.2015.04.037
Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis
Abstract
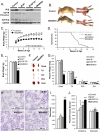
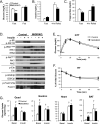
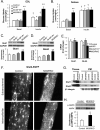
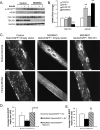
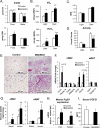
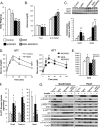
Insulin and insulin-like growth factor 1 (IGF-1) are major regulators of muscle protein and glucose homeostasis. To determine how these pathways interact, we generated mice with muscle-specific knockout of IGF-1 receptor (IGF1R) and insulin receptor (IR). These MIGIRKO mice showed >60% decrease in muscle mass. Despite a complete lack of insulin/IGF-1 signaling in muscle, MIGIRKO mice displayed normal glucose and insulin tolerance. Indeed, MIGIRKO mice showed fasting hypoglycemia and increased basal glucose uptake. This was secondary to decreased TBC1D1 resulting in increased Glut4 and Glut1 membrane localization. Interestingly, overexpression of a dominant-negative IGF1R in muscle induced glucose intolerance in MIGIRKO animals. Thus, loss of insulin/IGF-1 signaling impairs muscle growth, but not whole-body glucose tolerance due to increased membrane localization of glucose transporters. Nonetheless, presence of a dominant-negative receptor, even in the absence of functional IR/IGF1R, induces glucose intolerance, indicating that interactions between these receptors and other proteins in muscle can impair glucose homeostasis.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. - PMC - PubMed
-
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR042238/AR/NIAMS NIH HHS/United States
- K08 DK100543/DK/NIDDK NIH HHS/United States
- K08DK100543/DK/NIDDK NIH HHS/United States
- U24 DK100469/DK/NIDDK NIH HHS/United States
- P30 DK36836/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- R01AR42238/AR/NIAMS NIH HHS/United States
- R01 DK033201/DK/NIDDK NIH HHS/United States
- R37 DK031036/DK/NIDDK NIH HHS/United States
- R01 DK031036/DK/NIDDK NIH HHS/United States
- U24DK100469/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

