Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila
- PMID: 25981791
- PMCID: PMC4452410
- DOI: 10.1016/j.cub.2015.03.058
Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila
Abstract
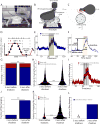
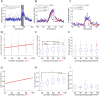
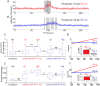
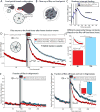
The neural circuit mechanisms underlying emotion states remain poorly understood. Drosophila offers powerful genetic approaches for dissecting neural circuit function, but whether flies exhibit emotion-like behaviors has not been clear. We recently proposed that model organisms may express internal states displaying "emotion primitives," which are general characteristics common to different emotions, rather than specific anthropomorphic emotions such as "fear" or "anxiety." These emotion primitives include scalability, persistence, valence, and generalization to multiple contexts. Here, we have applied this approach to determine whether flies' defensive responses to moving overhead translational stimuli ("shadows") are purely reflexive or may express underlying emotion states. We describe a new behavioral assay in which flies confined in an enclosed arena are repeatedly exposed to an overhead translational stimulus. Repetitive stimuli promoted graded (scalable) and persistent increases in locomotor velocity and hopping, and occasional freezing. The stimulus also dispersed feeding flies from a food resource, suggesting both negative valence and context generalization. Strikingly, there was a significant delay before the flies returned to the food following stimulus-induced dispersal, suggestive of a slowly decaying internal defensive state. The length of this delay was increased when more stimuli were delivered for initial dispersal. These responses can be mathematically modeled by assuming an internal state that behaves as a leaky integrator of stimulus exposure. Our results suggest that flies' responses to repetitive visual threat stimuli express an internal state exhibiting canonical emotion primitives, possibly analogous to fear in mammals. The mechanistic basis of this state can now be investigated in a genetically tractable insect species.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Simon HA. Motivational and Emotional Controls of Cognition. Psychol Rev. 1967;74:29–&. - PubMed
-
- Sloman A, Croucher M. Why robots will have emotions 1981
-
- Darwin C. The expression of the emotions in man and animals. London: J. Murray; 1872.
-
- Oatley K, Johnson-Laird PN. Towards a cognitive theory of emotions. Cogn emot. 1987;1:29–50.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

