Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials
- PMID: 25981812
- PMCID: PMC4489408
- DOI: 10.1016/S1470-2045(15)70168-3
Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials
Erratum in
-
Correction to Lancet Oncol 2015; 16: 634, 637.Lancet Oncol. 2015 Sep;16(9):e427. doi: 10.1016/S1470-2045(15)00238-7. Lancet Oncol. 2015. PMID: 26370351 No abstract available.
Abstract
Background: The standard of care for operable, stage I, non-small-cell lung cancer (NSCLC) is lobectomy with mediastinal lymph node dissection or sampling. Stereotactic ablative radiotherapy (SABR) for inoperable stage I NSCLC has shown promising results, but two independent, randomised, phase 3 trials of SABR in patients with operable stage I NSCLC (STARS and ROSEL) closed early due to slow accrual. We aimed to assess overall survival for SABR versus surgery by pooling data from these trials.
Methods: Eligible patients in the STARS and ROSEL studies were those with clinical T1-2a (<4 cm), N0M0, operable NSCLC. Patients were randomly assigned in a 1:1 ratio to SABR or lobectomy with mediastinal lymph node dissection or sampling. We did a pooled analysis in the intention-to-treat population using overall survival as the primary endpoint. Both trials are registered with ClinicalTrials.gov (STARS: NCT00840749; ROSEL: NCT00687986).
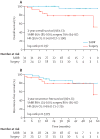
Findings: 58 patients were enrolled and randomly assigned (31 to SABR and 27 to surgery). Median follow-up was 40·2 months (IQR 23·0-47·3) for the SABR group and 35·4 months (18·9-40·7) for the surgery group. Six patients in the surgery group died compared with one patient in the SABR group. Estimated overall survival at 3 years was 95% (95% CI 85-100) in the SABR group compared with 79% (64-97) in the surgery group (hazard ratio [HR] 0·14 [95% CI 0·017-1·190], log-rank p=0·037). Recurrence-free survival at 3 years was 86% (95% CI 74-100) in the SABR group and 80% (65-97) in the surgery group (HR 0·69 [95% CI 0·21-2·29], log-rank p=0·54). In the surgery group, one patient had regional nodal recurrence and two had distant metastases; in the SABR group, one patient had local recurrence, four had regional nodal recurrence, and one had distant metastases. Three (10%) patients in the SABR group had grade 3 treatment-related adverse events (three [10%] chest wall pain, two [6%] dyspnoea or cough, and one [3%] fatigue and rib fracture). No patients given SABR had grade 4 events or treatment-related death. In the surgery group, one (4%) patient died of surgical complications and 12 (44%) patients had grade 3-4 treatment-related adverse events. Grade 3 events occurring in more than one patient in the surgery group were dyspnoea (four [15%] patients), chest pain (four [15%] patients), and lung infections (two [7%]).
Interpretation: SABR could be an option for treating operable stage I NSCLC. Because of the small patient sample size and short follow-up, additional randomised studies comparing SABR with surgery in operable patients are warranted.
Funding: Accuray Inc, Netherlands Organisation for Health Research and Development, NCI Cancer Center Support, NCI Clinical and Translational Science Award.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
SABR in early operable lung cancer: time for evidence.Lancet Oncol. 2015 Jun;16(6):597-8. doi: 10.1016/S1470-2045(15)70225-1. Epub 2015 May 13. Lancet Oncol. 2015. PMID: 25981817 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer.Lancet Oncol. 2015 Aug;16(8):e370-1. doi: 10.1016/S1470-2045(15)00036-4. Lancet Oncol. 2015. PMID: 26248836 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer.Lancet Oncol. 2015 Aug;16(8):e371-2. doi: 10.1016/S1470-2045(15)00063-7. Lancet Oncol. 2015. PMID: 26248837 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer.Lancet Oncol. 2015 Aug;16(8):e372-3. doi: 10.1016/S1470-2045(15)00123-0. Lancet Oncol. 2015. PMID: 26248838 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer.Lancet Oncol. 2015 Aug;16(8):e372. doi: 10.1016/S1470-2045(15)00091-1. Lancet Oncol. 2015. PMID: 26248839 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer.Lancet Oncol. 2015 Aug;16(8):e373-4. doi: 10.1016/S1470-2045(15)00145-X. Lancet Oncol. 2015. PMID: 26248840 No abstract available.
-
Surgery versus SABR for resectable non-small-cell lung cancer - Authors' reply.Lancet Oncol. 2015 Aug;16(8):e374-5. doi: 10.1016/S1470-2045(15)00154-0. Lancet Oncol. 2015. PMID: 26248841 No abstract available.
-
Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence?J Thorac Cardiovasc Surg. 2015 Sep;150(3):468-71. doi: 10.1016/j.jtcvs.2015.06.086. Epub 2015 Jul 14. J Thorac Cardiovasc Surg. 2015. PMID: 26259993 No abstract available.
-
SABR vs surgery for NSCLC in the media.Lancet Oncol. 2015 Sep;16(9):e422. doi: 10.1016/S1470-2045(15)00230-2. Lancet Oncol. 2015. PMID: 26370343 No abstract available.
-
[Randomised comparison of stereotactic body radiotherapy versus lobectomy in stage I NSCLC--level I evidence at last?].Strahlenther Onkol. 2015 Dec;191(12):988-90. doi: 10.1007/s00066-015-0905-4. Strahlenther Onkol. 2015. PMID: 26459463 German. No abstract available.
-
Stereotactic radiotherapy or surgery for early-stage non-small-cell lung cancer.Lancet Oncol. 2016 Feb;17(2):e41-e42. doi: 10.1016/S1470-2045(16)00026-7. Lancet Oncol. 2016. PMID: 26868347 No abstract available.
-
Stereotactic radiotherapy or surgery for early-stage non-small-cell lung cancer - Authors' reply.Lancet Oncol. 2016 Feb;17(2):e42-e43. doi: 10.1016/S1470-2045(16)00028-0. Lancet Oncol. 2016. PMID: 26868349 No abstract available.
References
-
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–31. - PubMed
-
- Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–92. - PubMed
-
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82:967–73. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

