Mitochondrial activity in gametes and transmission of viable mtDNA
- PMID: 25981894
- PMCID: PMC4435915
- DOI: 10.1186/s13062-015-0057-6
Mitochondrial activity in gametes and transmission of viable mtDNA
Abstract
Background: The retention of a genome in mitochondria (mtDNA) has several consequences, among which the problem of ensuring a faithful transmission of its genetic information through generations despite the accumulation of oxidative damage by reactive oxygen species (ROS) predicted by the free radical theory of ageing. A division of labour between male and female germ line mitochondria was proposed: since mtDNA is maternally inherited, female gametes would prevent damages by repressing oxidative phosphorylation, thus being quiescent genetic templates. We assessed mitochondrial activity in gametes of an unusual biological system (doubly uniparental inheritance of mitochondria, DUI), in which also sperm mtDNA is transmitted to the progeny, thus having to overcome the problem of maintaining genetic information viability while producing ATP for swimming.
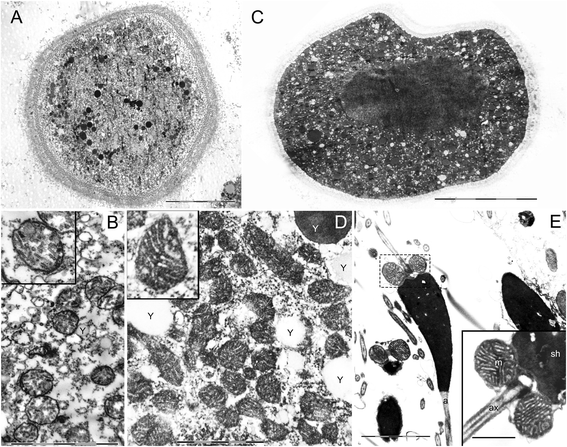
Results: Ultrastructural analysis shows no difference in the conformation of mitochondrial cristae in male and female mature gametes, while mitochondria in immature oocytes exhibit a simpler internal structure. Our data on transcriptional activity in germ line mitochondria show variability between sexes and different developmental stages, but we do not find evidence for transcriptional quiescence of mitochondria. Our observations on mitochondrial membrane potential are consistent with mitochondria being active in both male and female gametes.
Conclusions: Our findings and the literature we discussed may be consistent with the hypothesis that template mitochondria are not functionally silenced, on the contrary their activity might be fundamental for the inheritance mechanism. We think that during gametogenesis, fertilization and embryo development, mitochondria undergo selection for different traits (e.g. replication, membrane potential), increasing the probability of the transmission of functional organelles. In these phases of life cycle, the great reduction in mtDNA copy number per organelle/cell and the stochastic segregation of mtDNA variants would greatly improve the efficiency of selection. When a higher mtDNA copy number per organelle/cell is present, selection on mtDNA deleterious mutants is less effective, due to the buffering effect of wild-type variants. In our opinion, a combination of drift and selection on germ line mtDNA population, might be responsible for the maintenance of viable mitochondrial genetic information through generations, and a mitochondrial activity would be necessary for the selective process.
Figures





References
-
- Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5:123–35. - PubMed
-
- Breton S, Milani L, Ghiselli F, Guerra D, Stewart DT, Passamonti M. A resourceful genome: updating the functional repertoire and evolutionary role of animal mitochondrial DNAs. Trends Genet. 2014;30:555-64. - PubMed
-
- Allen JF. Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol. 1993;165:609–31. - PubMed
-
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

