Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases
- PMID: 25982012
- PMCID: PMC4998046
- DOI: 10.1016/j.lungcan.2015.04.004
Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases
Abstract
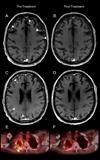
In-frame fusion KIF5B (the-kinesin-family-5B-gene)-RET transcripts have been characterized in 1-2% of non-small cell lung cancers and are known oncogenic drivers. The RET tyrosine kinase inhibitor, vandetanib, suppresses fusion-induced, anchorage-independent growth activity. In vitro studies have shown that vandetanib is a high-affinity substrate of breast cancer resistance protein (Bcrp1/Abcg2) but is not transported by P-glycoprotein (P-gp), limiting its blood-brain barrier penetration. A co-administration strategy to enhance the brain accumulation of vandetanib by modulating P-gp/Abcb1- and Bcrp1/Abcg2-mediated efflux with mTOR inhibitors, specifically everolimus, was shown to increase the blood-brain barrier penetration. We report the first bench-to-bedside evidence that RET inhibitor combined with an mTOR inhibitor is active against brain-metastatic RET-rearranged lung cancer and the first evidence of blood-brain barrier penetration. A 74-year-old female with progressive adenocarcinoma of the lung (wild-type EGFR and no ALK rearrangement) presented for therapy options. A deletion of 5'RET was revealed by FISH assay, indicating RET-gene rearrangement. Because of progressive disease in the brain, she was enrolled in a clinical trial with vandetanib and everolimus (NCT01582191). Comprehensive genomic profiling revealed fusion of KIF5B (the-kinesin-family-5B-gene) and RET, in addition to AKT2 gene amplification. After two cycles of therapy a repeat MRI brain showed a decrease in the intracranial disease burden and PET/CT showed systemic response as well. Interestingly, AKT2 amplification seen is a critical component of the PI3K/mTOR pathway, alterations of which has been associated with both de novo and acquired resistance to targeted therapy. The addition of everolimus may have both overcome the AKT2 amplification to produce a response in addition to its direct effects on the RET gene. Our case report forms the first evidence of blood-brain barrier penetration by vandetanib in combination with everolimus. Further research is required in this setting.
Keywords: Brain metastases; Everolimus; Exceptional responder; Lung cancer; Next generation sequencing; RET; Vandetanib; mTOR.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
SMA, CM and VAM are employees of and have equity interest in Foundation Medicine.
Figures

References
-
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. - PubMed
-
- Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121–126. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

