Nuclear transport factors: global regulation of mitosis
- PMID: 25982429
- PMCID: PMC4529785
- DOI: 10.1016/j.ceb.2015.04.012
Nuclear transport factors: global regulation of mitosis
Abstract
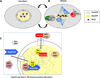
The unexpected repurposing of nuclear transport proteins from their function in interphase to an equally vital and very different set of functions in mitosis was very surprising. The multi-talented cast when first revealed included the import receptors, importin alpha and beta, the small regulatory GTPase RanGTP, and a subset of nuclear pore proteins. In this review, we report that recent years have revealed new discoveries in each area of this expanding story in vertebrates: (a) The cast of nuclear import receptors playing a role in mitotic spindle regulation has expanded: both transportin, a nuclear import receptor, and Crm1/Xpo1, an export receptor, are involved in different aspects of spindle assembly. Importin beta and transportin also regulate nuclear envelope and pore assembly. (b) The role of nucleoporins has grown to include recruiting the key microtubule nucleator - the γ-TuRC complex - and the exportin Crm1 to the mitotic kinetochores of humans. Together they nucleate microtubule formation from the kinetochores toward the centrosomes. (c) New research finds that the original importin beta/RanGTP team have been further co-opted by evolution to help regulate other cellular and organismal activities, ranging from the actual positioning of the spindle within the cell perimeter, to regulation of a newly discovered spindle microtubule branching activity, to regulation of the interaction of microtubule structures with specific actin structures. (d) Lastly, because of the multitudinous roles of karyopherins throughout the cell cycle, a recent large push toward testing their potential as chemotherapeutic targets has begun to yield burgeoning progress in the clinic.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Republished in
-
Reprint of "Nuclear transport factors: global regulation of mitosis".Curr Opin Cell Biol. 2015 Jun;34:122-34. doi: 10.1016/j.ceb.2015.07.005. Epub 2015 Jul 18. Curr Opin Cell Biol. 2015. PMID: 26196321 Review.
References
-
- Maresca TJ, Heald R. Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts. Methods Mol Biol. 2006;322:459–474. - PubMed
-
-
Kalab P, Pu RT, Dasso M. The ran GTPase regulates mitotic spindle assembly. Curr Biol. 1999;9:481–484.. • References [–7] represent the flurry of studies that first revealed karyopherin/RanGTP regulation as a cellular strategy for mitotic regulation.
-
-
- Zhang C, Hughes M, Clarke PR. Ran-GTP stabilises microtubule asters and inhibits nuclear assembly in Xenopus egg extracts. J Cell Sci. 1999;112(Pt 14):2453–2461. - PubMed
-
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell. 2001;104:95–106. - PubMed
-
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

