Stabilization of proteins in solid form
- PMID: 25982818
- PMCID: PMC4623959
- DOI: 10.1016/j.addr.2015.05.006
Stabilization of proteins in solid form
Abstract
Immunogenicity of aggregated or otherwise degraded protein delivered from depots or other biopharmaceutical products is an increasing concern, and the ability to deliver stable, active protein is of central importance. We review characterization approaches for solid protein dosage forms with respect to metrics that are intended to be predictive of protein stability against aggregation and other degradation processes. Each of these approaches is ultimately motivated by hypothetical connections between protein stability and the material property being measured. We critically evaluate correlations between these properties and stability outcomes, and use these evaluations to revise the currently standing hypotheses. Based on this we provide simple physical principles that are necessary (and possibly sufficient) for generating solid delivery vehicles with stable protein loads. Essentially, proteins should be strongly coupled (typically through H-bonds) to the bulk regions of a phase-homogeneous matrix with suppressed β relaxation. We also provide a framework for reliable characterization of solid protein forms with respect to stability.
Keywords: Aggregation; Dynamic stabilization; Lyophilization; Protein stability; Water substitution.
Published by Elsevier B.V.
Figures
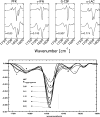
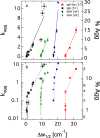

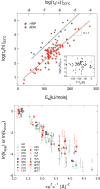

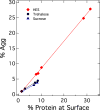
Comment in
-
Amorphous Pharmaceutical Solids.Adv Drug Deliv Rev. 2016 May 1;100:1-2. doi: 10.1016/j.addr.2016.04.011. Adv Drug Deliv Rev. 2016. PMID: 27153765 No abstract available.
References
-
- Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotech. 2000;18:24–25. - PubMed
-
- Costantino H, Langer R, Klibanov A. Moisture-Induced Aggregation of Lyophilized Insulin. Pharm Res. 1994;11:21–29. - PubMed
-
- Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D. Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. The Plant Journal. 1991;1:355–359. - PubMed
-
- Storey JM, Storey KB. Functional Metabolism: Regulation and Adaptation. John Wiley & Sons, Inc; 2004. pp. 473–503.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

