Reconstructing rare soil microbial genomes using in situ enrichments and metagenomics
- PMID: 25983722
- PMCID: PMC4415585
- DOI: 10.3389/fmicb.2015.00358
Reconstructing rare soil microbial genomes using in situ enrichments and metagenomics
Abstract
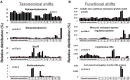
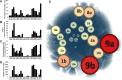
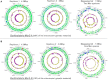
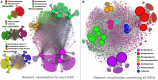
Despite extensive direct sequencing efforts and advanced analytical tools, reconstructing microbial genomes from soil using metagenomics have been challenging due to the tremendous diversity and relatively uniform distribution of genomes found in this system. Here we used enrichment techniques in an attempt to decrease the complexity of a soil microbiome prior to sequencing by submitting it to a range of physical and chemical stresses in 23 separate microcosms for 4 months. The metagenomic analysis of these microcosms at the end of the treatment yielded 540 Mb of assembly using standard de novo assembly techniques (a total of 559,555 genes and 29,176 functions), from which we could recover novel bacterial genomes, plasmids and phages. The recovered genomes belonged to Leifsonia (n = 2), Rhodanobacter (n = 5), Acidobacteria (n = 2), Sporolactobacillus (n = 2, novel nitrogen fixing taxon), Ktedonobacter (n = 1, second representative of the family Ktedonobacteraceae), Streptomyces (n = 3, novel polyketide synthase modules), and Burkholderia (n = 2, includes mega-plasmids conferring mercury resistance). Assembled genomes averaged to 5.9 Mb, with relative abundances ranging from rare (<0.0001%) to relatively abundant (>0.01%) in the original soil microbiome. Furthermore, we detected them in samples collected from geographically distant locations, particularly more in temperate soils compared to samples originating from high-latitude soils and deserts. To the best of our knowledge, this study is the first successful attempt to assemble multiple bacterial genomes directly from a soil sample. Our findings demonstrate that developing pertinent enrichment conditions can stimulate environmental genomic discoveries that would have been impossible to achieve with canonical approaches that focus solely upon post-sequencing data treatment.
Keywords: environmental genomics; metagenomics; phages; plasmids; rare biosphere; soil.
Figures




References
-
- Barrineau P., Gilbert P., Jackson W., Jones C., Summers A., Wisdom S. (1983). The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J. Mol. Appl. Genet. 2, 601–619. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

