Treatment for postpolio syndrome
- PMID: 25984923
- PMCID: PMC11236427
- DOI: 10.1002/14651858.CD007818.pub3
Treatment for postpolio syndrome
Abstract
Background: Postpolio syndrome (PPS) may affect survivors of paralytic poliomyelitis and is characterised by a complex of neuromuscular symptoms leading to a decline in physical functioning. The effectiveness of pharmacological treatment and rehabilitation management in PPS is not yet established. This is an update of a review first published in 2011.
Objectives: To systematically review the evidence from randomised and quasi-randomised controlled trials for the effect of any pharmacological or non-pharmacological treatment for PPS compared to placebo, usual care or no treatment.
Search methods: We searched the following databases on 21 July 2014: Cochrane Neuromuscular Disease Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and CINAHL Plus. We also checked reference lists of all relevant articles, searched the Database of Abstracts of Reviews of Effects (DARE), the Health Technology Assessment (HTA) Database and trial registers and contacted investigators known to be involved in research in this area.
Selection criteria: Randomised and quasi-randomised trials of any form of pharmacological or non-pharmacological treatment for people with PPS. The primary outcome was self perceived activity limitations and secondary outcomes were muscle strength, muscle endurance, fatigue, pain and adverse events.
Data collection and analysis: We used standard methodological procedures expected by The Cochrane Collaboration.
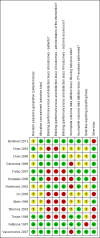
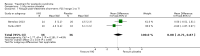
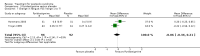
Main results: We included 10 pharmacological (modafinil, intravenous immunoglobulin (IVIg), pyridostigmine, lamotrigine, amantadine, prednisone) and three non-pharmacological (muscle strengthening, rehabilitation in a warm climate (that is temperature ± 25°C, dry and sunny) and a cold climate (that is temperature ± 0°C, rainy or snowy), static magnetic fields) studies with a total of 675 participants with PPS in this review. None of the included studies were completely free from any risk of bias, the most prevalent risk of bias being lack of blinding.There was moderate- and low-quality evidence that IVIg has no beneficial effect on activity limitations in the short term and long term, respectively, and inconsistency in the evidence for effectiveness on muscle strength. IVIg caused minor adverse events in a substantial proportion of the participants. Results of one trial provided very low-quality evidence that lamotrigine might be effective in reducing pain and fatigue, resulting in fewer activity limitations without generating adverse events. Data from two single trials suggested that muscle strengthening of thumb muscles (very low-quality evidence) and static magnetic fields (moderate-quality evidence) are safe and beneficial for improving muscle strength and pain, respectively, with unknown effects on activity limitations. Finally, there was evidence varying from very low quality to high quality that modafinil, pyridostigmine, amantadine, prednisone and rehabilitation in a warm or cold climate are not beneficial in PPS.
Authors' conclusions: Due to insufficient good-quality data and lack of randomised studies, it was impossible to draw definite conclusions about the effectiveness of interventions for PPS. Results indicated that IVIg, lamotrigine, muscle strengthening exercises and static magnetic fields may be beneficial but need further investigation to clarify whether any real and meaningful effect exists.
Conflict of interest statement
FK is involved in a RCT on the effectiveness of exercise therapy and cognitive behavioural therapy in PPS (Koopman 2014); its results are not yet published. Her work on the review was supported by a grant from Prinses Beatrix Spierfonds (The Dutch Public Fund for Neuromuscular Disorders)/ ZonMw (The Netherlands Organisation for Health Research and Development), Netherlands.
AB carried out a RCT on the effect of pyridostigmine in PPS (Horemans 2003). She is involved in Koopman 2014. Her work on the review was supported by a grant from Prinses Beatrix Spierfonds (The Dutch Public Fund for Neuromuscular Disorders)/ ZonMw (The Netherlands Organisation for Health Research and Development), Netherlands.
NEG was involved in a RCT on the effect of IVIg in PPS (Farbu 2007). He has received payment for scientific lectures and travel support to scientific meetings form the pharmaceutical companies Baxter, Octapharma and Merck Serono.
FN was an investigator on Horemans 2003. He is involved in Koopman 2014. He is also involved in a planned RCT on the effectiveness of IVIg (NCT02176863). He received study grants from the Netherlands Organisation for Health Research and Development and the Prinses Beatrix Spierfonds, Otto Bock, Fior & Gentz, OIM Orthopedie and for consultancy from Grifols Pharmaceuticals. All of these grants were paid to his institution.
MdV was an investigator on Horemans 2003. She is involved in Koopman 2014.
None of the review authors have financial conflicts of interest in the findings of this review.
Figures


















































Update of
-
Treatment for postpolio syndrome.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD007818. doi: 10.1002/14651858.CD007818.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 May 18;(5):CD007818. doi: 10.1002/14651858.CD007818.pub3. PMID: 21328301 Updated.
Similar articles
-
Treatment for postpolio syndrome.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD007818. doi: 10.1002/14651858.CD007818.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 May 18;(5):CD007818. doi: 10.1002/14651858.CD007818.pub3. PMID: 21328301 Updated.
-
Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD010369. doi: 10.1002/14651858.CD010369.pub2. Cochrane Database Syst Rev. 2017. PMID: 28084646 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2024 Dec 19;12(12):CD003200. doi: 10.1002/14651858.CD003200.pub9. Cochrane Database Syst Rev. 2024. PMID: 39697147
Cited by
-
Effects of muscle strengthening and cardiovascular fitness activities for poliomyelitis survivors: A systematic review and meta-analysis.J Rehabil Med. 2021 Apr 27;53(4):jrm00184. doi: 10.2340/16501977-2832. J Rehabil Med. 2021. PMID: 33876251 Free PMC article.
-
Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain.PLoS One. 2019 Jan 9;14(1):e0210418. doi: 10.1371/journal.pone.0210418. eCollection 2019. PLoS One. 2019. PMID: 30625201 Free PMC article.
-
Pyridostigmine: Postpoliomyelitis Syndrome.Hosp Pharm. 2016 May;51(5):367-9. doi: 10.1310/hpj5105-367. Hosp Pharm. 2016. PMID: 27303089 Free PMC article.
-
Health-related quality of life, self-reported impairments and activities of daily living in relation to muscle function in post-polio syndrome.J Patient Rep Outcomes. 2020 Jul 16;4(1):59. doi: 10.1186/s41687-020-00226-5. J Patient Rep Outcomes. 2020. PMID: 32676980 Free PMC article.
-
Sixty-nine years after surviving the polio epidemic.CMAJ. 2021 Dec 20;193(50):E1915-E1917. doi: 10.1503/cmaj.210985. CMAJ. 2021. PMID: 34930766 Free PMC article. No abstract available.
References
References to studies included in this review
Bertolasi 2013 {published and unpublished data}
-
- Bertolasi L, Frasson E, Turri M, Gajofatto A, Bordignon M, Zanolin E, et al. A randomized controlled trial of IV immunoglobulin in patients with postpolio syndrome. Journal of the Neurological Sciences 2013;330(1‐2):94‐9. [PUBMED: 23683859] - PubMed
Chan 2003 {published and unpublished data}
-
- Chan KM, Amirjani N, Sumrain M, Clarke A, Strohschein FJ. Randomized controlled trial of strength training in post‐polio patients. Muscle & Nerve 2003;27(3):332‐8. [PUBMED: 12635120] - PubMed
Chan 2006 {published and unpublished data}
-
- Chan KM, Strohschein FJ, Rydz D, Allidina A, Shuaib A, Westbury CF. Randomized controlled trial of modafinil for the treatment of fatigue in postpolio patients. Muscle & Nerve 2006;33(1):138‐41. [PUBMED: 16175627] - PubMed
Dinsmore 1995 {published and unpublished data}
-
- Dinsmore S, Dambrosia J, Dalakas MC. A double‐blind, placebo‐controlled trial of high‐dose prednisone for the treatment of post‐poliomyelitis syndrome. Annals of the New York Academy of Sciences 1995;753:303‐13. [PUBMED: 7611639] - PubMed
Farbu 2007 {published data only}
-
- Farbu E, Rekand T, Vik‐Mo E, Lygren H, Gilhus NE, Aarli JA. Post‐polio syndrome patients treated with intravenous immunoglobulin: a double‐blinded randomized controlled pilot study. European Journal of Neurology 2007;14(1):60‐5. [PUBMED: 16403993] - PubMed
Gonzalez 2006 {published and unpublished data}
-
- Gonzalez H, Sunnerhagen KS, Sjöberg I, Kaponides G, Olsson T, Borg K. Intravenous immunoglobulin for post‐polio syndrome: a randomised controlled trial. The Lancet Neurology 2006;5(6):493‐500. [PUBMED: 16713921] - PubMed
Horemans 2003 {published data only}
On 2005 {published data only}
-
- On AY, Oncu J, Uludag B, Ertekin C. Effects of lamotrigine on the symptoms and life qualities of patients with post polio syndrome: a randomized, controlled study. NeuroRehabilitation 2005;20(4):245‐51. [PUBMED: 16403993] - PubMed
Stein 1995 {published and unpublished data}
-
- Stein DP, Dambrosia JM, Dalakas MC. A double‐blind, placebo‐controlled trial of amantadine for the treatment of fatigue in patients with the post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:296‐302. [PUBMED: 7611638] - PubMed
Strumse 2003 {published and unpublished data}
-
- Strumse YA, Stanghelle JK, Utne L, Ahlvin P, Svendsby EK. Treatment of patients with postpolio syndrome in a warm climate. Disability and Rehabilitation 2003;25(2):77‐84. [PUBMED: 12554382] - PubMed
-
- Strumse YA, Stanghelle JK, Utne L, Ahlvin P, Svendsby EK. Treatment of patients with postpolio syndrome in warm climate. Tidsskrift for Den Norske Laegeforening 2001;121(17):2003‐7. [PUBMED: 11875895] - PubMed
Trojan 1999 {published data only}
-
- Trojan DA, Collet JP, Shapiro S, Jubelt B, Miller RG, Agre JC, et al. A multicenter, randomized, double‐blinded trial of pyridostigmine in postpolio syndrome. Neurology 1999;53(6):1225‐33. [PUBMED: 10522877] - PubMed
Vallbona 1997 {published data only}
-
- Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double‐blind pilot study. Archives of Physical Medicine & Rehabilitation 1997;78(11):1200‐3. [PUBMED: 9365349] - PubMed
Vasconcelos 2007 {published data only}
-
- Vasconcelos OM, Prokhorenko OA, Salajegheh MK, Kelley KF, Livornese K, Olsen CH, et al. Modafinil for treatment of fatigue in post‐polio syndrome: a randomized controlled trial. Neurology 2007;68(20):1680‐6. [PUBMED: 17502549] - PubMed
-
- Vasconcelos OM, Prokhorenko OA, Vo AH, Kelly KF, Dalakas MC, Lauro HS, et al. Controlled trial of modafinil for the treatment of fatigue in post‐polio syndrome. Neurology 2006;66(Suppl 2):A248. - PubMed
References to studies excluded from this review
Acler 2013 {published data only}
-
- Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post‐polio syndrome. Restorative Neurology and Neuroscience 2013;31(5):661‐8. - PubMed
Bruno 1996 {published data only}
-
- Bruno RL, Zimmerman JR, Creange SJ, Lewis T, Molzen T, Frick NM. Bromocriptine in the treatment of post‐polio fatigue: a pilot study with implications for the pathophysiology of fatigue. American Journal of Physical Medicine and Rehabilitation 1996;75(5):340‐7. [PUBMED: 8873700] - PubMed
Dean 1988 {published data only}
-
- Dean E, Ross J. Modified aerobic walking program: effect on patients with postpolio syndrome symptoms. Archives of Physical Medicine and Rehabilitation 1988;69(12):1033‐8. [PUBMED: 3214262] - PubMed
Dean 1991 {published data only}
-
- Dean E, Ross J. Effect of modified aerobic training on movement energetics in polio survivors. Orthopedics 1991;14(11):1243‐6. [PUBMED: 1758791] - PubMed
Ghahari 2010 {published and unpublished data}
-
- Ghahari S, Leigh Packer T, Passmore AE. Effectiveness of an online fatigue self‐management programme for people with chronic neurological conditions: a randomized controlled trial. Clinical Rehabilitation 2010;24(8):727‐44. [PUBMED: 20543022] - PubMed
-
- Ghahari S, Packer TL, Passmore AE. Development, standardisation and pilot testing of an online fatigue self‐management program. Disability and Rehabilitation 2009;31(21):1762‐72. [PUBMED: 19479510] - PubMed
Jones 1989 {published and unpublished data}
-
- Jones DR, Speier J, Canine K, Owen R, Stull GA. Cardiorespiratory responses to aerobic training by patients with postpoliomyelitis sequelae. JAMA 1989;261(22):3255‐8. [PUBMED: 2654435] - PubMed
Khan 2013 {published data only}
-
- Khan S, Gajapathi M, Pandit M. Additive effect of pulsed electromagnetic field therapy (PEMF) on hip flexor contracture in post polio syndrome (PPS). Indian Journal of Physiotherapy & Occupational Therapy 2013;7(2):196‐201.
Klein 2002 {published and unpublished data}
-
- Klein MG, Whyte J, Esquenazi A, Keenan MA, Costello R. A comparison of the effects of exercise and lifestyle modification on the resolution of overuse symptoms of the shoulder in polio survivors: a preliminary study. Archives of Physical Medicine and Rehabilitation 2002;83(5):708‐13. [PUBMED: 11994812] - PubMed
Kriz 1992 {published and unpublished data}
-
- Kriz JL, Jones DR, Speier JL, Canine JK, Owen RR, Serfass RC. Cardiorespiratory responses to upper extremity aerobic training by postpolio subjects. Archives of Physical Medicine and Rehabilitation 1992;73(1):49‐54. [PUBMED: 1729974] - PubMed
Miller 1997 {published and unpublished data}
-
- Miller RG, Gelinas DF, Kent‐Braun J, Dobbins T, Dao H, Dalakas M. The effect of recombinant insulin‐like growth factor 1 (rhIGF‐1), upon exercise induced fatigue and recovery in patients with post‐polio syndrome. Neurology 1997;48:A217. Abstract.
Oncu 2009 {published data only}
-
- Oncu J, Durmaz B, Karapolat H. Short‐term effects of aerobic exercise on functional capacity, fatigue, and quality of life in patients with post‐polio syndrome. Clinical Rehabilitation 2009;23(2):155‐63. [PUBMED: 19164403] - PubMed
Skough 2008 {published and unpublished data}
-
- Skough K, Krossén C, Heiwe S, Theorell H, Borg K. Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post‐polio: a pilot study. Journal of Rehabilitation Medicine 2008;40(9):773‐5. [PUBMED: 18843432] - PubMed
Skough 2011 {published data only}
-
- Skough K. Effects of resistance training in combination with IVIG treatment in patients with post‐polio. Journal of Rehabilitation Medicine. 1st European Polio Conference: ‘Post polio syndrome – a challenge of today'; 2011 August 31 – September 2; Copenhagen. 2011; Vol. Suppl 49:33.
-
- Skough K, Borg K, Henriksson M. Effects of resistance training in combination with IVIG treatment in patients with post‐polio. European Journal of Neurology. 15th Congress of the EFNS Budapest. 2011.
Willen 2001 {published and unpublished data}
-
- Willén C, Sunnerhagen KS, Grimby G. Dynamic water exercise in individuals with late poliomyelitis. Archives of Physical Medicine and Rehabilitation 2001;82(1):66‐72. [PUBMED: 11239288] - PubMed
References to studies awaiting assessment
ACTRN12612000552886 {published and unpublished data}
-
- ACTRN12612000552886. Oral supplementation of polio survivors with post polio syndrome or late effects of polio by coenzyme Q10 for relief of their excessive fatigue, as measured by fatigue questionnaires. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... (accessed 14 July 2014).
ISRCTN00378146 {published and unpublished data}
-
- ISRCTN00378146. Cost‐utility and efficacy of a home‐based exercise program in poliomyelitis survivors: paralytic poliomyelitis survivors with one (or two) lower limb affected more than twenty years ago, recruited through local association. http://www.controlled‐trials.com/ISRCTN00378146 (accessed 14 July 2014).
Koopman 2014 {published and unpublished data}
-
- Koopman FS, Beelen A, Gerrits KH, Bleijenberg G, Abma TA, Visser M, et al. Exercise therapy and cognitive behavioural therapy to improve fatigue, daily activity performance and quality of life in postpoliomyelitis syndrome: the protocol of the FACTS‐2‐PPS trial. BMC Neurology 2010;10:8. [PUBMED: 20082714] - PMC - PubMed
-
- Koopman FS, Voorn E, Brehm M, Beelen A, Nollet F. Exercise therapy and cognitive behavioural therapy to improve fatigue in post‐polio syndrome: preliminary results of a randomized controlled trial. Journal of Rehabilitation Medicine. 2nd European Polio Conference: 'Post‐Polio Syndrome Conference: a condition without boundaries’. 2014 June 25–27; Amsterdam. 2014; Vol. 46:589.
Murray 2014 {published and unpublished data}
-
- Murray D, Horgan F, Campion A, Vance R, Meldrum D, Hardiman O. The effects of a home‐based arm ergometry exercise programme on physical fitness, fatigue and activity in polio survivors; a randomised controlled trial. Journal of Rehabilitation Medicine. 2nd European Polio Conference: ‘Post‐Polio Syndrome Conference: a condition without boundaries’. 2014 June 25–27; Amsterdam. 2014; Vol. 46:589.
Silva 2014 {published and unpublished data}
-
- Moreira G, Silva T, Pradella‐Hallinan M, Quadros A, Oliveira A, Tufik S. Impact of BioceramicTM mattress on sleep of patients with postpolio syndrome and periodic leg movement. A randomized controlled study. Journal of Sleep Research. 20th Congress of the European Sleep Research Society. 2010 September 14‐18; Lisbon. 2010:335.
-
- Silva TM. Analyses of sleep characteristics in Post‐Polio Syndrome Patients. Journal of Rehabilitation Medicine. 1st European Polio Conference: ‘Post Polio Syndrome – a Challenge of Today'. 2011 August 31 – September 2; Copenhagen. 2011; Vol. Suppl 49:47.
References to ongoing studies
NCT01549847 {published data only}
-
- NCT01549847. A phase III, randomized, double blind, placebo controlled trial to evaluate the therapeutic effect of the association of L‐carnitine and piracetam as an adjuvant therapy in the treatment of weakness, muscle fatigue and muscle pain in the postpoliomyelitis syndrome. http://www.clinicaltrials.gov/ct2/results?term=NCT01549847 (accessed 14 July 2014).
NCT02089880 {published data only}
-
- NCT02089880. Micro‐processor controlled knee‐ankle‐foot orthosis (C‐brace) versus stance‐control knee‐ankle‐foot orthosis (SCO): functional outcomes in individuals with lower extremity impairment. http://www.clinicaltrials.gov/ct2/results?term=NCT02089880 (accessed 14 July 2014).
NCT02176863 {published data only}
-
- NCT02176863. Study of the efficacy and safety of immune globulin intravenous (human) Flebogamma® 5% DIF in patients with post‐polio syndrome. http://www.clinicaltrials.gov/ct2/show/record/NCT02176863?term=NCT02176863 (accessed 14 July 2014).
Additional references
Allen 1994
-
- Allen GM, Gandevia SC, Neering IR, Hickie I, Jones R, Middleton J. Muscle performance, voluntary activation and perceived effort in normal subjects and patients with prior poliomyelitis. Brain 1994;117:661‐70. [PUBMED: 7922455] - PubMed
Alvarez 2010
-
- Alvarez A, Kremer R, Weiss DR, Benedetti A, Haziza M, Trojan DA. Response of postpoliomyelitis patients to bisphosphonate treatment. Physical Medicine and Rehabilitation 2010;2(12):1094‐103. - PubMed
Bamford 1993
-
- Bamford CR, Montgomery EB Jr, Munoz JE, Stumpf C, Pry S, Namerow NS. Postpolio syndrome‐‐response to deprenyl (selegiline). The International Journal of Neuroscience 1993;71(1‐4):183‐8. [PUBMED: 8407144] - PubMed
Beelen 2003
-
- Beelen A, Nollet F, Visser M, Jong BA, Lankhorst GJ, Sargeant AJ. Quadriceps muscle strength and voluntary activation after polio. Muscle & Nerve 2003;28(2):218‐26. [PUBMED: 12872327] - PubMed
Bickerstaffe 2014
-
- Bickerstaffe A, Dijk JP, Beelen A, Zwarts MJ, Nollet F. Loss of motor unit size and quadriceps strength over 10 years in post‐polio syndrome. Clinical Neurophysiology 2014;125(6):1255‐60. - PubMed
Borg 1996
-
- Borg K. Post‐polio muscle dysfunction 29th ENMC workshop 14‐16 October 1994, Naarden, the Netherlands. Neuromuscular Disorders 1996;6(1):75‐80. [PUBMED: 8845722] - PubMed
Brehm 2007
-
- Brehm MA, Beelen A, Doorenbosch CA, Harlaar J, Nollet F. Effect of carbon‐composite knee‐ankle‐foot orthoses on walking efficiency and gait in former polio patients. Journal of Rehabilitation Medicine 2007;39(8):651‐7. [PUBMED: 17896058] - PubMed
Brogårdh 2010
-
- Brogårdh C, Flansbjer UB, Lexell J. No effects of whole‐body vibration training on muscle strength and gait performance in persons with late effects of polio: a pilot study. Archives of Physical Medicine and Rehabilitation 2010;91(9):1474‐7. - PubMed
Cup 2007
-
- Cup EH, Pieterse AJ, Broek‐Pastoor JM, Munneke M, Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Archives of Physical Medicine & Rehabilitation 2007;88(11):1452‐64. - PubMed
Dalakas 1986
-
- Dalakas MC, Elder G, Hallett M, Ravits J, Baker M, Papadopoulos N, et al. A long‐term follow‐up study of patients with post‐poliomyelitis neuromuscular symptoms. The New England Journal of Medicine 1986;314(15):959‐63. - PubMed
Dalakas 1988
-
- Dalakas MC. Morphologic changes in the muscles of patients with postpoliomyelitis neuromuscular symptoms. Neurology 1988;38(1):99‐104. [PUBMED: 3336469] - PubMed
Dalakas 1995
-
- Dalakas MC. The post‐polio syndrome as an evolved clinical entity. Definition and clinical description. Annals of the New York Academy of Sciences 1995;753:68‐80. [PUBMED: 7611661] - PubMed
Dalakas 1999
-
- Dalakas MC. Why drugs fail in postpolio syndrome: lessons from another clinical trial. Neurology 1999;53(6):1166‐7. [PUBMED: 10522866] - PubMed
Davidson 2009
-
- Davidson AC, Auyeung V, Luff R, Holland M, Hodgkiss A, Weinman J. Prolonged benefit in post‐polio syndrome from comprehensive rehabilitation: a pilot study. Disability and Rehabilitation 2009;31(4):309‐17. [PUBMED: 18608421] - PubMed
Deeks 2008
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:243‐96.
Dunn 1991
-
- Dunn MG. Post‐polio fatigue treated with amantadine. Archives of Neurology 1991;48(6):570. [PUBMED: 2039376] - PubMed
Farbu 2006
-
- Farbu E, Gilhus NE, Barnes MP, Borg K, Visser M, Driessen A, et al. EFNS guideline on diagnosis and management of post‐polio syndrome. Report of an EFNS task force. European Journal of Neurology 2006;13(8):795‐801. [PUBMED: 16879288] - PubMed
Farbu 2011
-
- Farbu E, Gilhus NE, Barnes MP, Borg K, Visser M, Howard R, et al. Post‐polio syndrome. In: Gilhus NE, Barnes MP, Brainin M editor(s). European Handbook of Neurological Management. 2nd Edition. Vol. 1, Blackwell Publishing Ltd, 2011:311‐9.
Gonzalez 2002
-
- Gonzalez H, Khademi M, Andersson M, Wallström E, Borg K, Olsson T. Prior poliomyelitis‐evidence of cytokine production in the central nervous system. Journal of the Neurological Sciences 2002;205(1):9‐13. - PubMed
Grimby 1989
-
- Grimby G, Einarsson G, Hedberg M, Aniansson A. Muscle adaptive changes in post‐polio subjects. Scandinavian Journal of Rehabilitation Medicine 1989;21(1):19‐26. [PUBMED: 2711135] - PubMed
Gupta 1994
-
- Gupta KL, Shetty KR, Agre JC, Cuisinier MC, Rudman IW, Rudman D. Human growth hormone effect on serum IGF‐I and muscle function in poliomyelitis survivors. Archives of Physical Medicine and Rehabilitation 1994;75(8):889‐94. [PUBMED: 8053796] - PubMed
Halstead 1985
-
- Halstead LS, Rossi CD. New problems in old polio patients: Results of a survey of 539 polio survivors. Orthopedics. 1985;8(7):845‐50. [PUBMED: 3867865] - PubMed
Halstead 1987
-
- Halstead LS, Rossi CD. Post‐polio syndrome: clinical experience with 132 consecutive outpatients. Birth Defects Original Article Series 1987;23(4):13‐26. [PUBMED: 3620612] - PubMed
Halstead 1991
-
- Halstead LS. Assessment and differential diagnosis for post‐polio syndrome. Orthopedics 1991;14(11):1209‐17. [PUBMED: 1758788] - PubMed
Heim 1997
-
- Heim M, Yaacobi E, Azaria M. A pilot study to determine the efficiency of lightweight carbon fibre orthoses in the management of patients suffering from post‐poliomyelitis syndrome. Clinical Rehabilitation 1997;11(4):302‐5. [PUBMED: 9408670] - PubMed
Higgins 2008
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:187‐241.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 2011
-
- Jensen MP, Alschuler KN, Smith AE, Verrall AM, Goetz MC, Molton IR. Pain and fatigue in persons with postpolio syndrome: independent effects on functioning. Archives of Physical Medicine and Rehabilitation 2011;92(11):1796‐801. - PubMed
Jubelt 1995
-
- Jubelt B, Salazar‐Grueso EF, Roos RP, Cashman NR. Antibody titer to the poliovirus in blood and cerebrospinal fluid of patients with post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:201‐7. [PUBMED: 7611629] - PubMed
Klefbeck 2000
-
- Klefbeck B, Lagerstrand L, Mattsson E. Inspiratory muscle training in patients with prior polio who use part‐time assisted ventilation. Archives of Physical Medicine and Rehabilitation 2000;81(8):1065‐71. [PUBMED: 10943756] - PubMed
Larsson Lund 2010
-
- Larsson Lund M, Lexell J. A positive turning point in life‐‐how persons with late effects of polio experience the influence of an interdisciplinary rehabilitation programme. Journal of Rehabilitation Medicine 2010;42(6):559‐65. - PubMed
Lefebre 2011
-
- Lefebre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
March of Dimes Foundation 2000
-
- March of Dimes Birth Defects Foundation. Identifying best practices in diagnosis & care. Proceedings of the International Conference on Post Polio Syndrome; 2000 May 19; Warm Springs, GA. New York. http://www.polioplace.org/sites/default/files/files/MOD‐%20Identifying.pdf. (accessed 14 August 2014).
McComas 1997
-
- McComas AJ, Quartly C, Griggs RC. Early and late losses of motor units after poliomyelitis. Brain 1997;120 Pt 8:1415‐21. - PubMed
Mizuno 1997
-
- Mizuno M, Quistorff B, Theorell H, Theorell M, Chance B. Effects of oral supplementation of coenzyme Q10 on 31P‐NMR detected skeletal muscle energy metabolism in middle‐aged post‐polio subjects and normal volunteers. Molecular Aspects of Medicine 1997;18 Suppl:S291‐8. [PUBMED: 9266539] - PubMed
Nollet 1999
-
- Nollet F, Beelen A, Prins MH, Visser M, Sargeant AJ, Lankhorst GJ, et al. Disability and functional assessment in former polio patients with and without postpolio syndrome. Archives of Physical Medicine and Rehabilitation 1999;80(2):136‐43. [PUBMED: 10025486] - PubMed
Nollet 2000
-
- Nollet F, Horemans H, Beelen A. A multicenter, randomized, double‐blinded trial of pyridostigmine in postpolio syndrome. Neurology 2000;55(6):899‐901. [PUBMED: 10994028] - PubMed
Nollet 2003
-
- Nollet F. Post‐polio syndrome. https://www.orpha.net/data/patho/GB/uk‐PP.pdf (accessed 14 August 2014).
Nollet 2003a
-
- Nollet F, Beelen A, Twisk JW, Lankhorst GJ, Visser M. Perceived health and physical functioning in postpoliomyelitis syndrome: a 6‐year prospective follow‐up study. Archives of Physical Medicine and Rehabilitation 2003;84(7):1048‐56. [PUBMED: 12881833] - PubMed
Nollet 2010
-
- Nollet F. Postpolio syndrome: unanswered questions regarding cause, course, risk factors, and therapies. The Lancet Neurology 2010;9(6):561‐3. [PUBMED: 20494317] - PubMed
Rekand 2004
-
- Rekand T, Kõrv J, Farbu E, Roose M, Gilhus NE, Langeland N, et al. Lifestyle and late effects after poliomyelitis. A risk factor study of two populations. Acta Neurologica Scandinavica 2004;109(2):120‐5. [PUBMED: 14705974] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seizert 1994
-
- Seizert BP, Speier JL, Canine K. Pyridostigmine effect on strength, endurance and fatigue in postpolio patients. Archives of Physical Medicine and Rehabilitation 1994;75:1049. Abstract.
Shetty 1995
-
- Shetty KR, Gupta KL, Agre JC, Rudman IW, Rudman D. Effect of human growth hormone on muscle function in post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:386‐9. [PUBMED: 7611652] - PubMed
Stolwijk‐Swuste 2005
-
- Stolwijk‐Swüste JM, Beelen A, Lankhorst GJ, Nollet F, CARPA Study Group. The course of functional status and muscle strength in patients with late‐onset sequelae of poliomyelitis: a systematic review. Archives of Physical Medicine and Rehabilitation 2005;86(8):1693‐701. [PUBMED: 16084828] - PubMed
Stolwijk‐Swuste 2010
-
- Stolwijk‐Swüste JM, Tersteeg I, Beelen A, Lankhorst GJ, Nollet F, CARPA Study Group. The impact of age and comorbidity on the progression of disability in late‐onset sequelae of poliomyelitis. Archives of Physical Medicine and Rehabilitation 2010;91(4):523‐8. - PubMed
Trojan 1993
-
- Trojan DA, Gendron D, Cashman NR. Stimulation frequency‐dependent neuromuscular junction transmission defects in patients with prior poliomyelitis. Journal of the Neurological Sciences 1993;118(2):150‐7. [PUBMED: 8229063] - PubMed
Trojan 1995
-
- Trojan DA, Cashman NR. An open trial of pyridostigmine in post‐poliomyelitis syndrome. Canadian Journal of Neurological Sciences 1995;22(3):223‐7. [PUBMED: 8529175] - PubMed
Wiechers 1981
-
- Wiechers DO, Hubbell SL. Late changes in the motor unit after acute poliomyelitis. Muscle & Nerve 1981;4(6):524‐8. [PUBMED: 6273721] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

