An early diagnostic tool for diabetic peripheral neuropathy in rats
- PMID: 25984949
- PMCID: PMC4436028
- DOI: 10.1371/journal.pone.0126892
An early diagnostic tool for diabetic peripheral neuropathy in rats
Erratum in
-
Correction: An Early Diagnostic Tool for Diabetic Peripheral Neuropathy in Rats.PLoS One. 2015 Jun 15;10(6):e0131144. doi: 10.1371/journal.pone.0131144. eCollection 2015. PLoS One. 2015. PMID: 26076138 Free PMC article. No abstract available.
Abstract
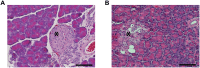
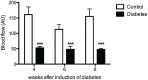
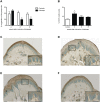
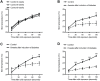
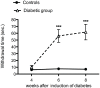
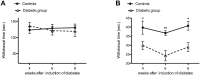
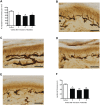
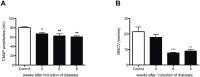
The skin's rewarming rate of diabetic patients is used as a diagnostic tool for early diagnosis of diabetic neuropathy. At present, the relationship between microvascular changes in the skin and diabetic neuropathy is unclear in streptozotocin (STZ) diabetic rats. The aim of this study was to investigate whether the skin rewarming rate in diabetic rats is related to microvascular changes and whether this is accompanied by changes observed in classical diagnostic methods for diabetic peripheral neuropathy. Computer-assisted infrared thermography was used to assess the rewarming rate after cold exposure on the plantar skin of STZ diabetic rats' hind paws. Peripheral neuropathy was determined by the density of intra-epidermal nerve fibers (IENFs), mechanical sensitivity, and electrophysiological recordings. Data were obtained in diabetic rats at four, six, and eight weeks after the induction of diabetes and in controls. Four weeks after the induction of diabetes, a delayed rewarming rate, decreased skin blood flow and decreased density of IENFs were observed. However, the mechanical hyposensitivity and decreased motor nerve conduction velocity (MNCV) developed 6 and 8 weeks after the induction of diabetes. Our study shows that the skin rewarming rate is related to microvascular changes in diabetic rats. Moreover, the skin rewarming rate is a non-invasive method that provides more information for an earlier diagnosis of peripheral neuropathy than the classical monofilament test and MNCV in STZ induced diabetic rats.
Conflict of interest statement
Figures

Similar articles
-
TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy.Mol Pain. 2018 Jan-Dec;14:1744806918789812. doi: 10.1177/1744806918789812. Epub 2018 Jul 3. Mol Pain. 2018. PMID: 29968518 Free PMC article.
-
Effects of U83836E on nerve functions, hyperalgesia and oxidative stress in experimental diabetic neuropathy.Life Sci. 2006 Jul 17;79(8):777-83. doi: 10.1016/j.lfs.2006.02.033. Epub 2006 Mar 31. Life Sci. 2006. PMID: 16581090
-
Reduced epidermal thickness, nerve degeneration and increased pain-related behavior in rats with diabetes type 1 and 2.J Chem Neuroanat. 2013 Nov;53:33-40. doi: 10.1016/j.jchemneu.2013.10.001. Epub 2013 Oct 12. J Chem Neuroanat. 2013. PMID: 24126225
-
The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy.Diabetologia. 2015 Apr;58(4):666-77. doi: 10.1007/s00125-014-3461-z. Epub 2014 Dec 16. Diabetologia. 2015. PMID: 25512003 Free PMC article. Review.
-
Pathophysiology and treatment of diabetic peripheral neuropathy: the case for diabetic neurovascular function as an essential component.Curr Diabetes Rev. 2006 May;2(2):131-45. doi: 10.2174/157339906776818569. Curr Diabetes Rev. 2006. PMID: 18220622 Review.
Cited by
-
Reconstruction of a 10-mm-long median nerve gap in an ischemic environment using autologous conduits with different patterns of blood supply: A comparative study in the rat.PLoS One. 2018 Apr 16;13(4):e0195692. doi: 10.1371/journal.pone.0195692. eCollection 2018. PLoS One. 2018. PMID: 29659600 Free PMC article.
-
TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy.Mol Pain. 2018 Jan-Dec;14:1744806918789812. doi: 10.1177/1744806918789812. Epub 2018 Jul 3. Mol Pain. 2018. PMID: 29968518 Free PMC article.
-
Effect of Azadirachta indica flower extract on functional recovery of sciatic nerve crush injury in rat models of DM.Exp Ther Med. 2019 Jan;17(1):541-550. doi: 10.3892/etm.2018.6931. Epub 2018 Nov 6. Exp Ther Med. 2019. PMID: 30651834 Free PMC article.
-
Correction: An Early Diagnostic Tool for Diabetic Peripheral Neuropathy in Rats.PLoS One. 2015 Jun 15;10(6):e0131144. doi: 10.1371/journal.pone.0131144. eCollection 2015. PLoS One. 2015. PMID: 26076138 Free PMC article. No abstract available.
-
Quantitative evaluation of skin disorders in type 1 diabetic mice by in vivo optical imaging.Biomed Opt Express. 2019 May 24;10(6):2996-3008. doi: 10.1364/BOE.10.002996. eCollection 2019 Jun 1. Biomed Opt Express. 2019. PMID: 31259069 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

