Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role
- PMID: 25985082
- PMCID: PMC4496700
- DOI: 10.3390/biom5020865
Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role
Abstract
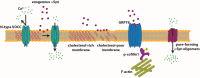
Mutations and duplication/triplication of the alpha-synuclein (αSyn)-coding gene have been found to cause familial Parkinson's disease (PD), while genetic polymorphisms in the region controlling the expression level and stability of αSyn have been identified as risk factors for idiopathic PD, pointing to the importance of wild-type (wt) αSyn dosage in the disease. Evidence that αSyn is present in the cerebrospinal fluid and interstitial brain tissue and that healthy neuronal grafts transplanted into PD patients often degenerate suggests that extracellularly-released αSyn plays a role in triggering the neurodegenerative process. αSyn's role in neurotransmission has been shown in various cell culture models in which the protein was upregulated or deleted and in knock out and transgenic animal, with different results on αSyn's effect on synaptic vesicle pool size and mobilization, αSyn being proposed as a negative or positive regulator of neurotransmitter release. In this review, we discuss the effect of αSyn on pre- and post-synaptic compartments in terms of synaptic vesicle trafficking, calcium entry and channel activity, and we focus on the process of exocytosis and internalization of αSyn and on the spreading of αSyn-driven effects due to the presence of the protein in the extracellular milieu.
Keywords: alpha-synuclein; calcium entry; exo/endocytosis; lipid microdomains; non-cell autonomous.
Figures


References
-
- Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Jensen P.H., Nielsen M.S., Jakes R., Dotti C.G., Goedert M. Binding of α-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J. Biol. Chem. 1998;273:26292–26294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

