MMP-2/9-Specific Activatable Lifetime Imaging Agent
- PMID: 25985157
- PMCID: PMC4481940
- DOI: 10.3390/s150511076
MMP-2/9-Specific Activatable Lifetime Imaging Agent
Abstract
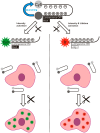
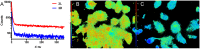
Optical (molecular) imaging can benefit from a combination of the high signal-to-background ratio of activatable fluorescence imaging with the high specificity of luminescence lifetime imaging. To allow for this combination, both imaging techniques were integrated in a single imaging agent, a so-called activatable lifetime imaging agent. Important in the design of this imaging agent is the use of two luminophores that are tethered by a specific peptide with a hairpin-motive that ensured close proximity of the two while also having a specific amino acid sequence available for enzymatic cleavage by tumor-related MMP-2/9. Ir(ppy)3 and Cy5 were used because in close proximity the emission intensities of both luminophores were quenched and the influence of Cy5 shortens the Ir(ppy)3 luminescence lifetime from 98 ns to 30 ns. Upon cleavage in vitro, both effects are undone, yielding an increase in Ir(ppy)3 and Cy5 luminescence and a restoration of Ir(ppy)3 luminescence lifetime to 94 ns. As a reference for the luminescence activation, a similar imaging agent with the more common Cy3-Cy5 fluorophore pair was used. Our findings underline that the combination of enzymatic signal activation with lifetime imaging is possible and that it provides a promising method in the design of future disease specific imaging agents.
Keywords: FRET; MMP; enzymatic activation; fluorescence; iridium; lifetime imaging; luminescence.
Figures





References
-
- Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005;11:227–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

