L-acetylcarnitine for treating fragile X syndrome
- PMID: 25985235
- PMCID: PMC10849109
- DOI: 10.1002/14651858.CD010012.pub2
L-acetylcarnitine for treating fragile X syndrome
Abstract
Background: People with fragile X syndrome (FXS) have an intellectual dysfunction that can range from very mild to severe. Symptoms can include speech and language delays and behavioural difficulties such as aggression or self injurious behaviours, emotional lability, and anxiety-related problems (for example obsessive-compulsive symptoms and perseverative behaviours). In some cases, affected people may have an additional diagnosis of attention deficit hyperactivity disorder or an autism spectrum disorder.
Objectives: To review the efficacy and safety of L-acetylcarnitine in improving the psychological, intellectual, and social performance of people with FXS.
Search methods: In May 2015 we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, Web of Science, and two other databases. We also searched three trials registers, four theses databases, and the reference lists of relevant studies and reviews.
Selection criteria: Randomised controlled trials (RCTs) that assessed the efficacy of L-acetylcarnitine, at any dose, in people of any age diagnosed with FXS compared with placebo.
Data collection and analysis: For each trial, two review authors independently extracted data on the children included and interventions compared, and assessed the risk of bias of the studies across the following domains: randomisation sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other potential sources of bias.
Main results: We found only two RCTs that compared oral L-acetylcarnitine (LAC) with oral placebo in children with FXS. The studies included a total of 83 participants, all of them male, who were treated and followed for one year. The age of participants at the start of treatment ranged from 6 to 13 years, with a mean age of 9 years. Neither study provided information on randomisation, allocation concealment procedures, or blinding of outcome assessment, and we received no responses from the authors we emailed for clarification. We therefore rated studies as being at unclear risk of bias on these domains. We judged both studies to be at low risk of bias for blinding of participants and personnel, incomplete outcome data, and selective reporting, but to be at high risk of other bias, as at least one study was funded by a drug company, and in both studies people working for the company were part of the research team.We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate the quality of the available evidence. Overall, the quality of the evidence was low due to the imprecision of results and high risk of other bias.Regarding the primary outcome of psychological and learning capabilities, both studies assessed the effect of interventions on children's verbal and non-verbal intellectual functioning using the Wechsler Intelligence Scale for Children - Revised. The authors did not provide detailed data on those results but said that they found no important differences between treatment and placebo.Both studies evaluated the impact of the treatment on hyperactive behaviour using the Conners' Abbreviated Parent-Teacher Questionnaire. In one study, teachers' assessments of the children found no clear evidence of a difference (mean difference (MD) 0.50, 95% confidence interval (CI) -5.08 to 6.08, n = 51; low-quality evidence). The other study stated that there were no differences between treated and untreated participants, but did not provide detailed data for inclusion in the meta-analysis.Parents' assessments favoured LAC in one study (MD -0.57, 95% CI -0.94 to -0.19, n = 17; low-quality evidence), but not in the other (MD -2.80, 95% CI -7.61 to 2.01, n = 51; low-quality evidence), though changes were not large enough to be considered clinically relevant.Regarding social skills, one study reported no clear evidence of a difference in Vineland Adaptive Behavior composite scores (MD 8.20, 95% CI -0.02 to 16.42, n = 51; low-quality evidence), yet results in the socialisation domain favoured LAC (MD 11.30, 95% CI 2.52 to 20.08, n = 51; low-quality evidence).Both studies assessed the safety of the active treatment and recorded no side effects. Neither of the included studies assessed the secondary outcome of caregiver burden.
Authors' conclusions: Low-quality evidence from two small trials showed that when compared to placebo, LAC may not improve intellectual functioning or hyperactive behaviour in children with FXS.
Conflict of interest statement
José‐Ramón Rueda ‐ none known. Virginia Guillén ‐ none known. Javier Ballesteros ‐ none known. Maria‐Isabel Tejada ‐ none known. Ivan Solà ‐ none known.
Figures


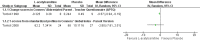
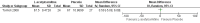
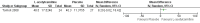
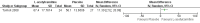



Update of
References
References to studies included in this review
Torrioli 1999 {published data only (unpublished sought but not used)}
-
- Torrioli MG, Vernacotola S, Mariotti P, Bianchi E, Calvani M, Gaetano A, et al. Double‐blind, placebo‐controlled study of L‐acetylcarnitine for the treatment of hyperactive behavior in fragile X syndrome. American Journal of Medical Genetics 1999;87(4):366‐8. - PubMed
Torrioli 2008 {published data only (unpublished sought but not used)}
-
- Calvani M, D'Iddio S, Gaetano A, Mariotti P, Mosconi L, Pomponi MG, et al. L‐acetylcarnitine treatment on fragile X patients hyperactive behaviour [El tratamiento con L‐acetilcarnitina del comportamiento hiperactivo de pacientes con el síndrome X frágil]. Revista de Neurologia 2001;33 Suppl 1:65‐70. - PubMed
-
- Torrioli MG, Vernacotola S, Peruzzi L, Tabolacci E, Mila M, Militerni R, et al. A double‐blind, parallel, multicenter comparison of L‐acetylcarnitine with placebo on the attention deficit hyperactivity disorder in fragile X syndrome boys. American Journal of Medical Genetics 2008;146A(7):803‐12. - PubMed
References to studies excluded from this review
Abbasi 2011a {published data only}
-
- Abbasi SH, Heidari S, Mohammadi MR, Tabrizi M, Ghaleiha A, Akhondzadeh S. Acetyl‐L‐carnitine as an adjunctive therapy in the treatment of attention‐deficit/hyperactivity disorder in children and adolescents: a placebo‐controlled trial. Child Psychiatry and Human Development 2011;42(3):367‐75. - PubMed
Arnold 2007 {published data only}
-
- Arnold LE, Amato A, Bozzolo H, Hollway J, Cook A, Ramadan Y, et al. Acetyl‐L‐carnitine (ALC) in attention‐deficit/hyperactivity disorder: a multi‐site, placebo‐controlled pilot trial. Journal of Child and Adolescent Psychopharmacology 2007;17(6):791‐802. - PubMed
Van Oudheusden 2002 {published data only}
-
- Oudheusden LJ, Scholte HR. Efficacy of carnitine in the treatment of children with attention‐deficit hyperactivity disorder. Prostaglandins Leukotrienes, and Essential Fatty Acids 2002;67(1):33‐8. - PubMed
Additional references
Abbasi 2011b
-
- Abbasi SH, Heidari S, Mohammadi MR, Tabrizi M, Ghaleiha A, Akhondzadeh S. Acetyl‐L‐carnitine as an adjunctive therapy in the treatment of attention‐deficit/hyperactivity disorder in children and adolescents: a placebo‐controlled trial. Child Psychiatry and Human Development 2011;42(3):367‐75. - PubMed
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington, DC: American Psychiatric Association, 2000.
Bender 1938
-
- Bender LA. A Visual Motor Gestalt Test and Its Clinical Use. New York: American Orthopsychiatry Association, 1938.
Benneto 2002
-
- Benneto L, Pennington BF. Neuropsychologie. In: Hagerman RJ, Hagerman PJ editor(s). Fragile X Syndrome, Diagnosis, Treatment and Research. 3rd Edition. Baltimore: Johns Hopkins University Press, 2002:206‐48.
Biederman 2004
-
- Biederman J, Faraone SV, Monuteaux MC, Grossbard JR. How informative are parent reports of attention‐deficit/hyperactivity disorder symptoms for assessing outcome in clinical trials of long‐acting treatments? A pooled analysis of parents' and teachers' reports. Pediatrics 2004;113(6):1667‐71. - PubMed
Calvani 2001
-
- Calvani M, D'Iddio S, Gaetano A, Mariotti P, Mosconi L, Pomponi MG, et al. L‐acetylcarnitine treatment on fragile X patients hyperactive behaviour [El tratamiento con L‐acetilcarnitina del comportamiento hiperactivo de pacientes con el síndrome X frágil]. Revista de Neurologia 2001;33 Suppl 1:65‐70. - PubMed
Chonchaiya 2009
Clifford 2007
-
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders 2007;37(4):738‐47. - PubMed
Conners 1990
-
- Conners CK. Conners' Rating Scales Revised ‐ Technical Manual. New York: Multi‐Health Systems, Inc, 1990.
Conners 1998
-
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257‐68. - PubMed
Crawford 2001
Dell'Anna 1997
-
- Dell'Anna E, Iuvone L, Calzolari S, Geloso MC. Effect of acetyl‐L carnitine on hyperactivity and spatial memory deficits of rats exposed to neonatal anoxia. Neuroscience Letters 1997;223(3):201‐5. - PubMed
Doll 1965
-
- Doll EA. Vineland Social Maturity Scale: Condensed Manual of Directions. Circle Pines, MN: American Guidance Service, Inc., 1965.
Fu 1991
-
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991;67(6):1047‐58. - PubMed
Gorini 1999
-
- Gorini A, D'Angelo A, Villa RF. Energy metabolism of synaptosomal subpopulations from different neuronal systems of rat hippocampus: effect of L‐acetylcarnitine administration in vivo. Neurochemical Research 1999;24(5):617‐24. - PubMed
Grade Working Group 2004
Hagerman 2008
Hagerman 2009
Hall 2009
Harris 2008
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne AC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hudson 2003
Jacquemont 2003
Pascale 2003
Pettegrew 1995
-
- Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl‐L‐carnitine in Alzheimer’s disease. Neurobiology of Aging 1995;16(1):1‐4. - PubMed
Pettegrew 2000
-
- Pettegrew JW, Levine J, McClure RJ. Acetyl‐L‐carnitine physical‐chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Molecular Psychiatry 2000;5(6):616‐32. - PubMed
Pomponi 1994
-
- Pomponi MG, Neri G. Butyrate and acetyl‐carnitine inhibit the cytogenetic expression of the fragile X in vitro. American Journal of Medical Genetics 1994;51(4):447‐50. - PubMed
Rodriguez‐Revenga 2013
-
- Rodriguez‐Revenga L, Madrigal I, Blanch‐Rubió J, Elurbe DM, Docampo E, Collado A, et al. Screening for the presence of FMR1 premutation alleles in women with fibromyalgia. Gene 2013;512(2):305‐8. - PubMed
Rueda 2009
Song 2003
-
- Song FJ, Barton P, Sleightholme V, Yao GL, Fry‐Smith A. Screening for fragile X syndrome: a literature review and modelling study. Health Technology Assessment 2003;7(16):1‐106. - PubMed
Tabolacci 2005
-
- Tabolacci E, Pietrobono R, Moscato U, Oostra BA, Chiurazzi P, Neri G. Differential epigenetic modifications in the FMR1 gene of the fragile X syndrome after reactivating pharmacological treatments. European Journal of Human Genetics 2005;13(5):641‐8. - PubMed
Tassone 2000
Thomson 2009a
Thomson 2009b
Toth 1993
-
- Toth E, Harsing LG Jr, Sershen H, Ramacci MT, Lajtha A. Effect of acetyl‐L‐carnitine on extracellular amino acid levels in vivo in rat brain regions. Neurochemical Research 1993;18(5):573‐8. - PubMed
Verkerk 1991
-
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR‐1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991;65(5):905‐14. - PubMed
Wechsler 2004
-
- Wechsler D. The Wechsler Intelligence Scale for Children. 4th Edition. London: Pearson Assessment, 2004.
Wittenberger 2007
-
- Wittenberger MD, Hagerman RJ, Sherman SL, McConkie‐Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertility and Sterility 2007;87(3):456‐65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

