T cells in the control of organ-specific autoimmunity
- PMID: 25985270
- PMCID: PMC4497750
- DOI: 10.1172/JCI78089
T cells in the control of organ-specific autoimmunity
Abstract
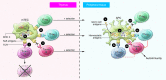
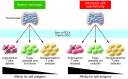
Immune tolerance is critical to the avoidance of unwarranted immune responses against self antigens. Multiple, non-redundant checkpoints are in place to prevent such potentially deleterious autoimmune responses while preserving immunity integral to the fight against foreign pathogens. Nevertheless, a large and growing segment of the population is developing autoimmune diseases. Deciphering cellular and molecular pathways of immune tolerance is an important goal, with the expectation that understanding these pathways will lead to new clinical advances in the treatment of these devastating diseases. The vast majority of autoimmune diseases develop as a consequence of complex mechanisms that depend on genetic, epigenetic, molecular, cellular, and environmental elements and result in alterations in many different checkpoints of tolerance and ultimately in the breakdown of immune tolerance. The manifestations of this breakdown are harmful inflammatory responses in peripheral tissues driven by innate immunity and self antigen-specific pathogenic T and B cells. T cells play a central role in the regulation and initiation of these responses. In this Review we summarize our current understanding of the mechanisms involved in these fundamental checkpoints, the pathways that are defective in autoimmune diseases, and the therapeutic strategies being developed with the goal of restoring immune tolerance.
Figures