Phosphatidylinositol 3-kinase signaling determines kidney size
- PMID: 25985273
- PMCID: PMC4497754
- DOI: 10.1172/JCI78945
Phosphatidylinositol 3-kinase signaling determines kidney size
Abstract
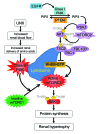
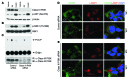
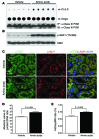
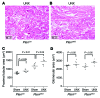
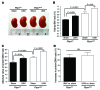
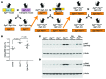
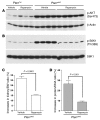
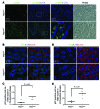
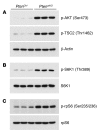
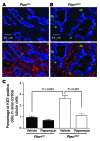
Kidney size adaptively increases as mammals grow and in response to the loss of 1 kidney. It is not clear how kidneys size themselves or if the processes that adapt kidney mass to lean body mass also mediate renal hypertrophy following unilateral nephrectomy (UNX). Here, we demonstrated that mice harboring a proximal tubule-specific deletion of Pten (Pten(ptKO)) have greatly enlarged kidneys as the result of persistent activation of the class I PI3K/mTORC2/AKT pathway and an increase of the antiproliferative signals p21(Cip1/WAF) and p27(Kip1). Administration of rapamycin to Pten(ptKO) mice diminished hypertrophy. Proximal tubule-specific deletion of Egfr in Pten(ptKO) mice also attenuated class I PI3K/mTORC2/AKT signaling and reduced the size of enlarged kidneys. In Pten(ptKO) mice, UNX further increased mTORC1 activation and hypertrophy in the remaining kidney; however, mTORC2-dependent AKT phosphorylation did not increase further in the remaining kidney of Pten(ptKO) mice, nor was it induced in the remaining kidney of WT mice. After UNX, renal blood flow and amino acid delivery to the remaining kidney rose abruptly, followed by increased amino acid content and activation of a class III PI3K/mTORC1/S6K1 pathway. Thus, our findings demonstrate context-dependent roles for EGFR-modulated class I PI3K/mTORC2/AKT signaling in the normal adaptation of kidney size and PTEN-independent, nutrient-dependent class III PI3K/mTORC1/S6K1 signaling in the compensatory enlargement of the remaining kidney following UNX.
Figures




Comment in
-
Kidney growth and hypertrophy: the role of mTOR and vesicle trafficking.J Clin Invest. 2015 Jun;125(6):2267-70. doi: 10.1172/JCI81508. Epub 2015 May 18. J Clin Invest. 2015. PMID: 25985272 Free PMC article.
-
Growth and development: Determinants of kidney size.Nat Rev Nephrol. 2015 Jul;11(7):387. doi: 10.1038/nrneph.2015.86. Epub 2015 Jun 2. Nat Rev Nephrol. 2015. PMID: 26035772 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

