Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity
- PMID: 25985354
- PMCID: PMC6273193
- DOI: 10.3390/molecules20058499
Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity
Abstract
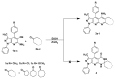
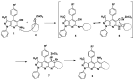
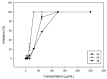
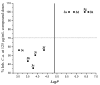
The microwave assisted reaction between heterocyclic o-aminonitriles 1 and cyclic ketones 2 catalyzed by zinc chloride led to new series of pyrazolo[3,4-b] [1,8]naphthyridin-5-amines 3 in good yields. This procedure provides several advantages such as being environmentally friendly, high yields, simple work-up procedure, broad scope of applicability and the protocol provides an alternative for the synthesis of pyrazolonaphthyridines. The whole series showed antifungal activities against Candida albicans and Cryptococcus neoformans standardized strains, being compounds with a 4-p-tolyl substituent of the naphthyridin scheleton (3a, 3d and 3g), the most active ones mainly against C. albicans, which appear to be related to their comparative hydrophobicity. Among them, 3d, containing a cyclohexyl fused ring, showed the best activity. The anti-Candida activity was corroborated by testing the three most active compounds against clinical isolates of albicans and non-albicans Candida strains. These compounds were also screened by the US National Cancer Institute (NCI) for their ability to inhibit 60 different human tumor cell lines. Compounds 3a and 3e showed remarkable antitumor activity against cancer cell lines, with the most important GI50 values ranging from 0.62 to 2.18 μM.
Keywords: Candida albicans; Cryptococcus neoformans; antifungal activity; antitumoral activity; microwave irradiation; pyrazolonaphthyridines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vanov A.S., Tugusheva N.Z., Granik V.G. Benzo[b]naphthyridines. Russ. Chem. Rev. 2005;74:915–936. doi: 10.1070/RC2005v074n10ABEH001196. - DOI
-
- Litvinov V.P. Chemistry and biological activities of 1,8-naphthyridines. Russ. Chem. Rev. 2004;73:637–669. doi: 10.1070/RC2004v073n07ABEH000856. - DOI
-
- Litvinov V.P., Roman S.V., Dyachenko V.D. Naphthyridines. Structure, physicochemical properties and general methods of synthesis. Russ. Chem. Rev. 2000;69:201–220. doi: 10.1070/RC2000v069n03ABEH000553. - DOI
-
- Roma G., Grossi G., Braccio M.D., Piras D., Ballabeni V., Tognolini M., Bertoni S., Barocelli E. 1,8-Naphthyridines VII. New substituted 5-amino[1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides and their isosteric analogues, exhibiting notable anti-inflammatory and/or analgesic activities, but no acute gastrolesivity. Eur. J. Med. Chem. 2008;43:1665–1680. doi: 10.1016/j.ejmech.2007.10.010. - DOI - PubMed
-
- Roma G., Braccio M.D., Grossi G., Mattioli F., Ghia M. 1,8-Naphthyridines IV. 9-Substituted N,N-dialkyl-5-(alkylamino or cycloalkylamino) [1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur. J. Med. Chem. 2000;35:1021–1035. doi: 10.1016/S0223-5234(00)01175-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

