KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis
- PMID: 25985364
- PMCID: PMC4552085
- DOI: 10.1038/nm.3866
KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis
Erratum in
-
Corrigendum: KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis.Nat Med. 2016 Feb;22(2):217. doi: 10.1038/nm0216-217a. Nat Med. 2016. PMID: 26845408 Free PMC article. No abstract available.
Abstract
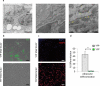
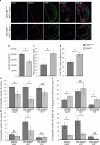
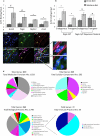
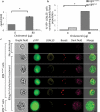
Previous studies investigating the role of smooth muscle cells (SMCs) and macrophages in the pathogenesis of atherosclerosis have provided controversial results owing to the use of unreliable methods for clearly identifying each of these cell types. Here, using Myh11-CreER(T2) ROSA floxed STOP eYFP Apoe(-/-) mice to perform SMC lineage tracing, we find that traditional methods for detecting SMCs based on immunostaining for SMC markers fail to detect >80% of SMC-derived cells within advanced atherosclerotic lesions. These unidentified SMC-derived cells exhibit phenotypes of other cell lineages, including macrophages and mesenchymal stem cells (MSCs). SMC-specific conditional knockout of Krüppel-like factor 4 (Klf4) resulted in reduced numbers of SMC-derived MSC- and macrophage-like cells, a marked reduction in lesion size, and increases in multiple indices of plaque stability, including an increase in fibrous cap thickness as compared to wild-type controls. On the basis of in vivo KLF4 chromatin immunoprecipitation-sequencing (ChIP-seq) analyses and studies of cholesterol-treated cultured SMCs, we identified >800 KLF4 target genes, including many that regulate pro-inflammatory responses of SMCs. Our findings indicate that the contribution of SMCs to atherosclerotic plaques has been greatly underestimated, and that KLF4-dependent transitions in SMC phenotype are critical in lesion pathogenesis.
Figures


References
-
- Saffitz JE, Schwartz CJ. Coronary atherosclerosis and thrombosis underlying acute myocardial infarction. Cardiol. Clin. 1987;5:21–30. - PubMed
-
- Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat. Med. 2002;8:1257–1262. - PubMed
-
- Falk E, Nakano M, Benton JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur. Heart J. 2012 - PubMed
-
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000;20:1262–1275. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- HL087867/HL/NHLBI NIH HHS/United States
- R37 HL057353/HL/NHLBI NIH HHS/United States
- P30 CA044579/CA/NCI NIH HHS/United States
- HL112904/HL/NHLBI NIH HHS/United States
- R00 HL112904/HL/NHLBI NIH HHS/United States
- K99 HL112904/HL/NHLBI NIH HHS/United States
- R01 HL087867/HL/NHLBI NIH HHS/United States
- R01 HL088554/HL/NHLBI NIH HHS/United States
- T32 HL007284/HL/NHLBI NIH HHS/United States
- R01 HL057353/HL/NHLBI NIH HHS/United States
- R01 HL121008/HL/NHLBI NIH HHS/United States
- R01 HL098538/HL/NHLBI NIH HHS/United States
- T32 CA009109/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

