National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report
- PMID: 25985921
- PMCID: PMC4506719
- DOI: 10.1016/j.bbmt.2015.05.004
National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report
Abstract
Treatment of chronic graft-versus-host disease is intended to produce a sustainable benefit by reducing symptom burden, controlling objective manifestations of disease activity, preventing damage and impairment, and improving overall survival without causing disproportionate harms related to the treatment itself. Successful management can control the disease until systemic treatment is no longer needed. The complexity of the disease, the extended duration of follow-up needed to observe disease resolution and withdrawal of immunosuppressive treatment, and the lack of fully developed shorter term endpoints impede progress in the field. Identification and characterization of primary endpoints demonstrating clinical benefit without requiring years of follow-up is urgently needed, with the understanding that clinical benefit encompasses not only the self-evident benefit of the primary endpoint but also any other associated benefits. This report discusses regulatory considerations, eligibility criteria, the value of controlled trial designs, the merits of proposed primary endpoints, and key considerations elaborated from experience and progress during the past decade. The report concludes by mapping an overall approach that could support and lead to maximally informative clinical trials, especially those that seek to demonstrate clinical benefit along a pathway to regulatory review and approval.
Keywords: Allogeneic hematopoietic cell transplantation; Chronic graft-versus-host disease; Clinical trials; Consensus; Design; Guidelines.
Copyright © 2015 American Society for Blood and Marrow Transplantation. All rights reserved.
Figures
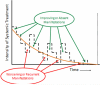
 ).
).References
-
- Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft- versus-host disease. Biol Blood Marrow Transplant. 2001;7:265–273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

