Glia Disease and Repair-Remyelination
- PMID: 25986556
- PMCID: PMC4484968
- DOI: 10.1101/cshperspect.a020594
Glia Disease and Repair-Remyelination
Abstract
The inability of the mammalian central nervous system (CNS) to undergo spontaneous regeneration has long been regarded as a central tenet of neurobiology. However, although this is largely true of the neuronal elements of the adult mammalian CNS, save for discrete populations of granular neurons, the same is not true of its glial elements. In particular, the loss of oligodendrocytes, which results in demyelination, triggers a spontaneous and often highly efficient regenerative response, remyelination, in which new oligodendrocytes are generated and myelin sheaths are restored to denuded axons. Yet, remyelination in humans is not without limitation, and a variety of demyelinating conditions are associated with sustained and disabling myelin loss. In this review, we will review the biology of remyelination, including the cells and signals involved; describe when remyelination occurs and when and why it fails and the consequences of its failure; and discuss approaches for therapeutically enhancing remyelination in demyelinating diseases of both children and adults, both by stimulating endogenous oligodendrocyte progenitor cells and by transplanting these cells into demyelinated brain.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
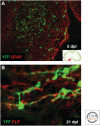
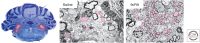

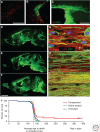
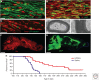
References
-
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. 2007. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6: 793–810. - PubMed
-
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JPY. 2001. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4: 1116–1122. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
