Environmental Sensing in Actinobacteria: a Comprehensive Survey on the Signaling Capacity of This Phylum
- PMID: 25986905
- PMCID: PMC4518836
- DOI: 10.1128/JB.00176-15
Environmental Sensing in Actinobacteria: a Comprehensive Survey on the Signaling Capacity of This Phylum
Abstract
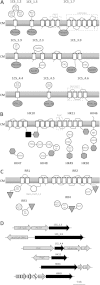
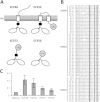
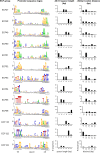
Signal transduction is an essential process that allows bacteria to sense their complex and ever-changing environment and adapt accordingly. Three distinct major types of signal-transducing proteins (STPs) can be distinguished: one-component systems (1CSs), two-component systems (2CSs), and extracytoplasmic-function σ factors (ECFs). Since Actinobacteria are particularly rich in STPs, we comprehensively investigated the abundance and diversity of STPs encoded in 119 actinobacterial genomes, based on the data stored in the Microbial Signal Transduction (MiST) database. Overall, we observed an approximately linear correlation between the genome size and the total number of encoded STPs. About half of all membrane-anchored 1CSs are protein kinases. For both 1CSs and 2CSs, a detailed analysis of the domain architectures identified novel proteins that are found only in actinobacterial genomes. Many actinobacterial genomes are particularly enriched for ECFs. As a result of this study, almost 500 previously unclassified ECFs could be classified into 18 new ECF groups. This comprehensive survey demonstrates that actinobacterial genomes encode previously unknown STPs, which may represent new mechanisms of signal transduction and regulation. This information not only expands our knowledge of the diversity of bacterial signal transduction but also provides clear and testable hypotheses about their mechanisms, which can serve as starting points for experimental studies.
Importance: In the wake of the genomic era, with its enormous increase in the amount of available sequence information, the challenge has now shifted toward making sense and use of this treasure chest. Such analyses are a prerequisite to provide meaningful information that can help guide subsequent experimental efforts, such as mechanistic studies on novel signaling strategies. This work provides a comprehensive analysis of signal transduction proteins from 119 actinobacterial genomes. We identify, classify, and describe numerous novel and conserved signaling devices. Hence, our work serves as an important resource for any researcher interested in signal transduction of this important bacterial phylum, which contains organisms of ecological, biotechnological, and medical relevance.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

