EMT, CTCs and CSCs in tumor relapse and drug-resistance
- PMID: 25986923
- PMCID: PMC4484413
- DOI: 10.18632/oncotarget.4037
EMT, CTCs and CSCs in tumor relapse and drug-resistance
Abstract
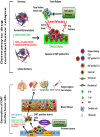
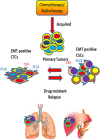
Tumor relapse and metastasis are the primary causes of poor survival rates in patients with advanced cancer despite successful resection or chemotherapeutic treatment. A primary cause of relapse and metastasis is the persistence of cancer stem cells (CSCs), which are highly resistant to chemotherapy. Although highly efficacious drugs suppressing several subpopulations of CSCs in various tissue-specific cancers are available, recurrence is still common in patients. To find more suitable therapy for relapse, the mechanisms underlying metastasis and drug-resistance associated with relapse-initiating CSCs need to be identified. Recent studies in circulating tumor cells (CTCs) of some cancer patients manifest phenotypes of both CSCs and epithelial-mesenchymal transition (EMT). These patients are unresponsive to standard chemotherapies and have low progression free survival, suggesting that EMT-positive CTCs are related to co-occur with or transform into relapse-initiating CSCs. Furthermore, EMT programming in cancer cells enables in the remodeling of extracellular matrix to break the dormancy of relapse-initiating CSCs. In this review, we extensively discuss the association of the EMT program with CTCs and CSCs to characterize a subpopulation of patients prone to relapses. Identifying the mechanisms by which EMT-transformed CTCs and CSCs initiate relapse could facilitate the development of new or enhanced personalized therapeutic regimens.
Keywords: CSCs; CTCs; EMT; clinical trials; tumor relapse.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

