EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-κB signaling pathway
- PMID: 25987128
- PMCID: PMC4546460
- DOI: 10.18632/oncotarget.3691
EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-κB signaling pathway
Abstract
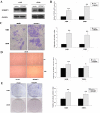
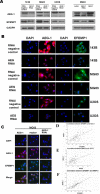
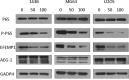
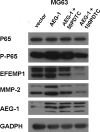
The role of epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) in osteosarcoma remains unknown. Then applying EFEMP1 siRNA, plasmids transfection and adding purified EFEMP1 protein in human osteosarcoma cell lines, and using immunohistochemistry on 113 osteosarcoma tissues, demonstrated that EFEMP1 was a poor prognostic indicator of osteosarcoma; EFEMP1 was specifically upregulated in osteosarcoma and associated with invasion and metastasis in vitro and in vivo. At the same time, we found a direct regulatory effect of EFEMP1 on MMP-2. Moreover, we firstly found the marked induction of EFEMP1 by oncogenic AEG-1. And EFEMP1 expression was inhibited by the selective inhibitor of NF-κB (PDTC) in osteosarcoma cells. Then we thought that NF-κB pathways might be one of the effective ways which EFEMP1 was induced by AEG-1. Thus, we suggested that EFEMP1 played a part as the mediator between AEG-1 and MMP-2. And NF-κB signaling pathway played an important role in this process. In summary, EFEMP1 was associated with invasion, metastasis and poor prognosis of osteosarcoma patients. EFEMP1 might indirectly enhance the expression of MMP-2, providing a potential explanation for the role of AEG-1 in metastasis. NF-κB pathways might be one of the effective ways which EFEMP1 was induced by AEG-1.
Keywords: AEG-1; EFEMP1; MMP-2; NF-κB; osteosarcoma.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures





References
-
- Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–489. - PubMed
-
- Stone EM, Lotery AJ, Munier FL, Héon E, Piguet B, Guymer RH, Vandenburgh K, Cousin P, Nishimura D, Swiderski RE, Silvestri G, Mackey DA, Hageman GS, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. - PubMed
-
- Obaya AJ, Rua S, Moncada-Pazos A, Cal S. The dual role of fibulins in tumorigenesis. Cancer Lett. 2012;325:132–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

